Figure 2. Hh distributions in the neural tube of the mouse embryo and model for signaling in a ciliated mouse cell.
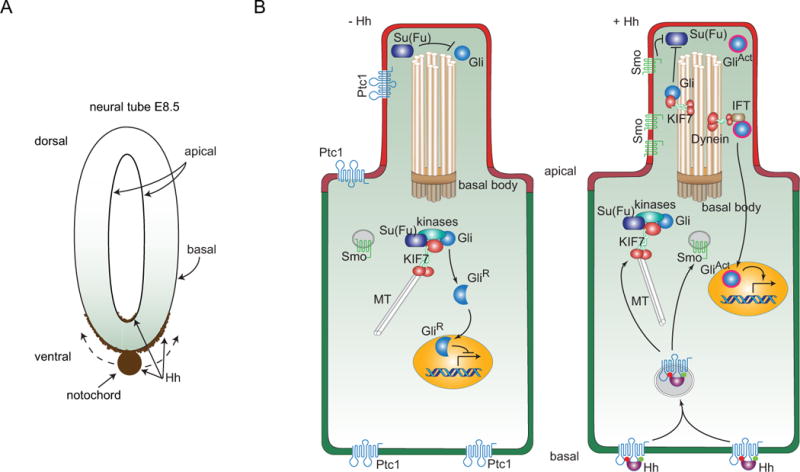
(A) Drawing depitcs Hh expression (brown) in the notochord of an E8.5 mouse embryo. Hh expressed in the notochord moves dorsally and distributes along the basal surface of the neural tube; some accumulates at the apical surface. (B) A subset of the components involved in Hh signal transduction is depicted in a cell prior to receipt of Hh (−Hh; left), and in a cell active for Hh signal transduction (+ Hh; right). The primary cilium contains a 9+0 bundle of doublet microtubules that extend from a basal body, and ferry components of Hh signal transduction with microtubule-binding KIF7 and Dynein motor proteins. The Ptc1 receptor (blue) is present in the apical plasma membrane, and in in non-signaling cells, in the plasma membrane of the primary cilium. Smo is only present in the plasma membrane of the primary cilium in Hh-signaling cells. In non-signaling cells, Smo is in intracellular vesicles and Gli is present both in the primary cilium where its inactive state is Su(Fu)-dependent and in the cytoplasm. In the cytoplasm it is associated with KIF7, Su(Fu) and several kinases and is processed to a proteolyzed repressor form that translocates to the nucleus. In signaling cells, shown as receiving Hh at the basal membrane, intracellular vesicles containing Ptc1 and Hh form, the process that generates GliR is inhibited, and in the primary cilium, Su(Fu)-dependent inactivation of Gli is inhibited and the transcriptional activator form of Gli is generated.
