Abstract
Objective
To determine predictors of early recovery of functional swallow in patients who had gastrostomy (percutaneous endoscopic gastrostomy [PEG]) placement for dysphagia and were discharged to inpatient rehabilitation (IPR) after stroke.
Methods
A retrospective study of prospectively identified patients with acute ischemic and hemorrhagic stroke from July 2008 to August 2012 was conducted. Patients who had PEG during stroke admission and were discharged to IPR, were studied. We compared demographics, stroke characteristics, severity of dysphagia, stroke admission events and medications in patients who remained PEG-dependent after IPR with those who recovered functional swallow.
Results
Patients who remained PEG dependent were significantly older (73 vs. 54 years, p=0.009). Recovery of swallow was more frequent for hemorrhagic stroke patients (80% vs. 47%, p=0.079). Age, adjusting for side of stroke (odds ratio [OR], 0.89; 95% confidence interval [CI], 0.82-0.98; p=0.016) and left-sided strokes, adjusting for age (OR, 15.15; 95% CI, 1.32-173.34; p=0.028) were significant predictors of swallow recovery. Patients who recovered swallowing by discharge from IPR were more likely to be discharged home compared to those who remained PEG-dependent (90% vs. 42%, p=0.009).
Conclusion
Younger age and left-sided stroke may be predictive factors of early recovery of functional swallow in patients who received PEG. Prospective validation is important as avoidance of unnecessary procedures could reduce morbidity and healthcare costs.
Keywords: Gastrostomy, Acute ischemic stroke, Rehabilitation, Dysphagia
INTRODUCTION
Dysphagia is prevalent among individuals with acute stroke. Studies suggest that dysphagia occurs in >42% of patients with acute stroke within a median of 3 days from stroke diagnosis. Dysphagia contributes significantly to stroke patients' overall morbidity and mortality; many of these patients regain functional swallowing spontaneously over time, but in some, the disability is long-term [1,2]. The temporal profile of swallowing recovery in patients with hemispheric infarcts remains poorly delineated. Variables that influence the recovery of swallowing function have not been well established. Bihemispheric infarcts, right hemisphere infarcts, dysarthria, intubation, the National Institute of Health Stroke Scale (NIHSS) ≥12, aspiration during videofluoroscopic swallowing study, and age greater than 52 years were significant predictors of persistent dysphagia [3,4,5,6].
Patients with persistent dysphagia require a long-term treatment modality for nutrition and hydration in order to be discharged home to inpatient rehabilitation (IPR) or to long-term care facilities. Gastrostomy (percutaneous endoscopic gastrostomy [PEG]) placement has become a preferred method for long-term feeding in patients with dysphagia incurred from stroke. Compared with nasogastric tube (NGT) feeding, PEG reduced intervention failures (e.g., feeding interruption, blocking or leakage of the tube, non-adherence to treatment and gastrointestinal bleeding), and had higher feed delivery and albumin concentration [7,8].
Previous studies have shown that early PEG placement (within 2 weeks) is effective to support nutritional and hydration needs as many patients with dysphagia have protracted oral feeding difficulties [9]. Despite the high mortality seen in the first few months after PEG insertion, some of the stroke patients that survive long-term have been able to attain a reasonable level of function in their daily activities [9]. Improvement in swallow function and eating often leads to PEG tube removal. Previous research has shown that up to one-third of the PEG tubes were removed before the patients were discharged from rehabilitation and 75% were removed by 1 year [10,11].
Prediction of recovery of functional swallowing may allow setting expectations for patients and family members when PEG is recommended, and deferral of PEG in cases where recovery is likely within the subacute phase of stroke rehabilitation. We sought to determine the predictors of early recovery of functional swallowing among stroke patients discharged to IPR centers. We have provided additional evidence that younger age, left sided strokes, and hemorrhagic strokes are associated with more functional swallowing recovery and have explored additional predictive factors in our patient population.
MATERIALS AND METHODS
We retrospectively reviewed consecutive patients with acute stroke presenting at our medical center from July 2008 to August 2012. Upon hospital admission, all patients received a dysphagia screen administered by the intensive care unit nursing staff. Within 24 hours of the admission, a bedside clinical swallow assessment by a speech and language therapist was performed, unless the patient was unable to participate. The second screening method was videofluoroscopic swallowing assessment, referred to as modified barium swallow study or MBSS; this was administered unless the patient was unable to participate or a diet was recommended based on lack of aspiration predictors on the bedside clinical swallow assessment. The decision for PEG insertion was individualized to each patient through the interdisciplinary evaluations that indicated enteral tube feeding was necessary to avoid further loss of body weight, to correct significant nutritional deficiencies, to rehydrate the patient, and to stop the related deterioration in the quality of life of the patient due to inadequate or unsafe oral nutritional intake. The interdisciplinary evaluations included sequential neurological assessments of the patient's ability/inability to participate in swallow assessments; sequential assessments of swallow function on clinical/bedside and/or MBSS with the speech-language pathologist; sequential adequacy of nutritional assessments with a dietician; and gastroenterology consult and/or general surgery consult to assess for patient's surgical candidacy for PEG insertion.
We identified patients who had PEG placement and the discharge disposition of these patients. We reviewed the discharge summaries from the IPR admission for all the patients who went to IPR with a PEG tube and identified patients who regained functional swallowing and patients who remained PEG dependent.
We collected information about age, gender, race, exposure to thrombolytic therapy, intubation, type of stroke (ischemic and hemorrhagic), localization of the stroke (right, left, bilateral, cortical, subcortical), length of hospitalization, antidepressant and neurostimulant treatment and stroke-related complications, including urinary tract infection and pneumonia. We compared demographics, stroke risk factors and characteristics, NIHSS scores on admission and at discharge, stroke admission events and discharge medications in patients who remained PEG-dependent after IPR with those who recovered functional swallowing. All NIHSS examinations were performed by NIHSS-certified healthcare providers. We also compared IPR length of stay and disposition after IPR in those who remained PEG-dependent after IPR with those who recovered functional swallowing.
Statistics
Baseline characteristics were compared between groups using Student t-test or Wilcoxon rank sum (for data not normally distributed) for the continuous variables and Pearson chi-square with Fisher exact test where appropriate for categorical variables. Crude and adjusted logistic regression models were used to assess predictors of PEG placement and recovery of swallow ability. As this was an exploratory analysis, no adjustments were made for multiple comparisons [12]. All tests were performed at the alpha 0.05 level.
The retrospective chart review was approved by the Institutional Review Board (IRB) at Tulane University (IRB Protocol No. 302012).
RESULTS
Patient demographics
Of the 1,364 patients who presented with acute stroke to Tulane University Medical Center in the 50-month period, 7.6% (n=103) received PEG during their acute hospital period. One-third (34/103) of patients who received PEG were discharged to IPR. One patient recovered a functional swallow prior to discharge to IPR; the remaining 33 patients were dependent on nutrition and hydration administered via PEG. Of the remaining 72 patients who received PEG, 13 (18.1%) were discharged to skilled nursing facilities, 27 (37.5%) were discharged to a nursing home, 15 (20.8%) were discharged to long-term acute care facilities, 6 (8.3%) went home with custodial care, 6 (8.3%) were discharged to a hospice, and 2 (2.8%) died prior to discharge.
Characteristics of PEG dependence
Recovery of swallow and discontinuation of PEG dependence could not be determined for one of the 33 PEG patients who were discharged to IPR, so this patient was excluded from further analyses. Of the remaining 32 patients, 56.2% (18/32) were female and 75.0% (24/32) were black. Hemorrhagic stroke was associated with significantly higher odds of having PEG placed (odds ratio [OR], 3.99; 95% confidence interval [CI], 2.58-6.18; p<0.001).
In our sample, 62.5% (20/32) of the followed patients successfully regained swallowing by discharge from the IPR. Although patients who regained functional swallowing were significantly younger (54 vs. 73 years, p=0.009), patients who remained PEG dependent reported significantly higher alcohol misuse history than patients who recovered swallowing (25.0% vs. 5.0%, p=0.011). There were no differences in race, gender or other demographic characteristics (Table 1). As shown in Table 2, patient age as a continuous variable is a significant predictor of recovering swallowing ability (OR, 0.93; 95% CI 0.87-0.99; p=0.0151). In the multivariable model adjusting for side of stroke, the odds of recovering swallowing function were lower in older individuals (Hosmer-Lemeshow goodness-of-fit p=0.2643; OR, 0.89; 95% CI, 0.82-0.98; p=0.016). We were not able to adjust for any other factors in the model due to the models failing to converge with additional covariates.
Table 1.
Comparison of PEG dependent and PEG independent groups baseline characteristics
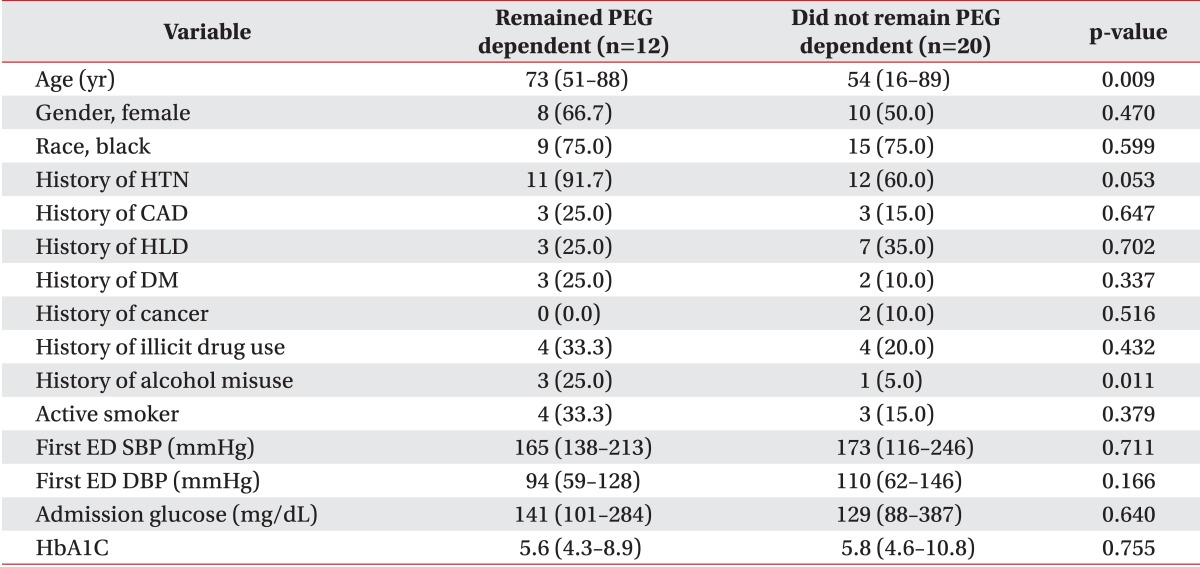
Values are presented as median (range) or number (%).
PEG, percutaneous endoscopic gastrostomy; HTN, hypertension; CAD, coronary artery disease; HLD, hyperlipidemia; DM, diabetes mellitus; ED, emergency department; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Table 2.
Odds of PEG recovery
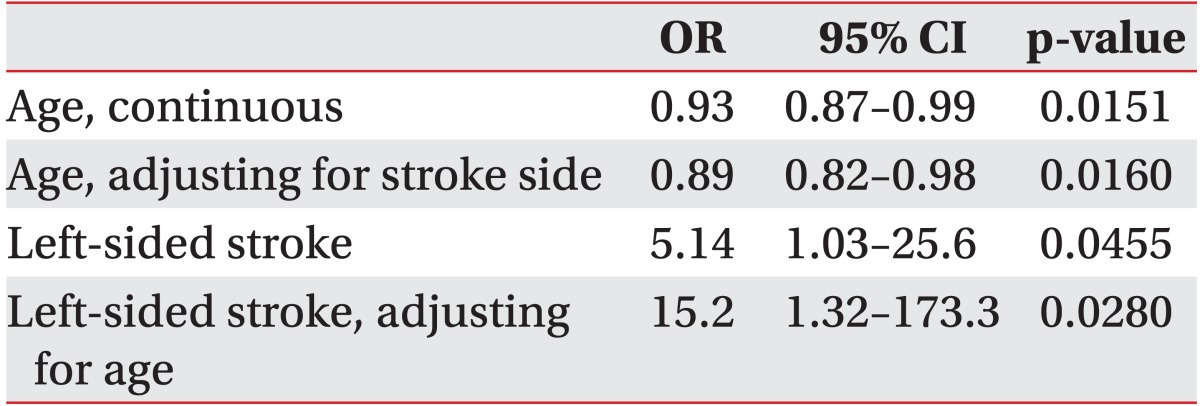
PEG, percutaneous endoscopic gastrostomy; OR, odds ratio; CI, confidence interval.
Stroke characteristics
As shown in Table 3, there was no statistically significant difference in stroke severity when patients who did recover swallow were compared to those who did not (median NIHSS 20 did recover vs. 19 did not recover, p=0.779). Aphasia, dysarthria, facial droop, neglect at baseline and NIHSS at discharge to IPR showed no statistically significant difference among patients who regained their swallowing function and those who did not.
Table 3.
Comparison of PEG dependent and PEG independent groups stroke characteristics
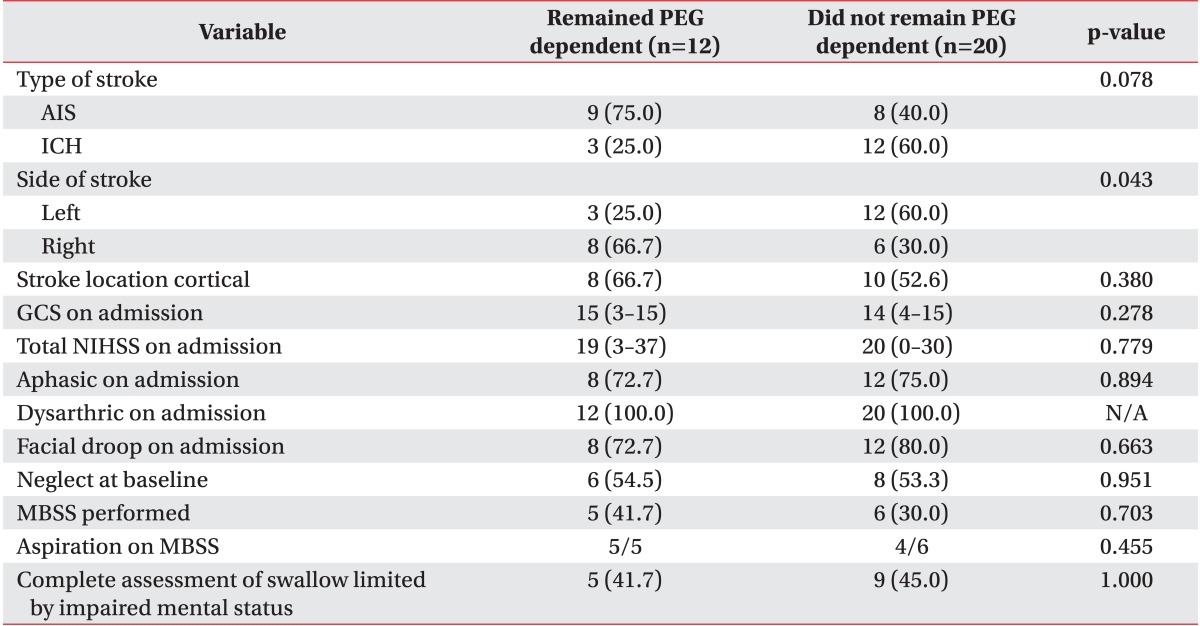
Values are presented as number (%) or median (range).
PEG, percutaneous endoscopic gastrostomy; AIS, acute ischemic stroke; ICH, intracerebral hemorrhage; GCS, Glasgow Coma Scale; NIHSS, National Institute of Health Stroke Scale; MBSS, modified barium swallow study; N/A, not available.
Although not statistically significant, recovery of swallow was more frequent for intracranial hemorrhage when compared to ischemic stroke (80.0% vs. 47.0%, p=0.079) (Table 3). PEG dependence occurred more frequently in patients with right hemispheric strokes (66.7% vs. 30.0%, p=0.043). The proportions of cortical and subcortical strokes were similar in patients who recovered and patients who did not (52.6% vs. 66.7%, p=0.380). Left-sided strokes had increased odds of recovering swallow function (OR, 5.14; 95% CI, 1.03-25.6; p=0.0455).
Acute stroke admission events
There was no statistically significant difference in the length of hospital stay for acute stroke (median length of stay for patients who recovered swallowing was 22 vs. 20 for those who remained PEG dependent, p=0.198).
There was no statistically significant difference in frequency of urinary tract infection, pneumonia, seizures, surgical procedures or neurological deterioration (Table 4). Intubation frequency 50.0% among patients who recovered swallow vs. 58.3% of those who remained PEG dependent was not statistically significantly different.
Table 4.
Comparison of inpatient complications and outcomes in patients who remained PEG dependent and patients who did not remain PEG dependent
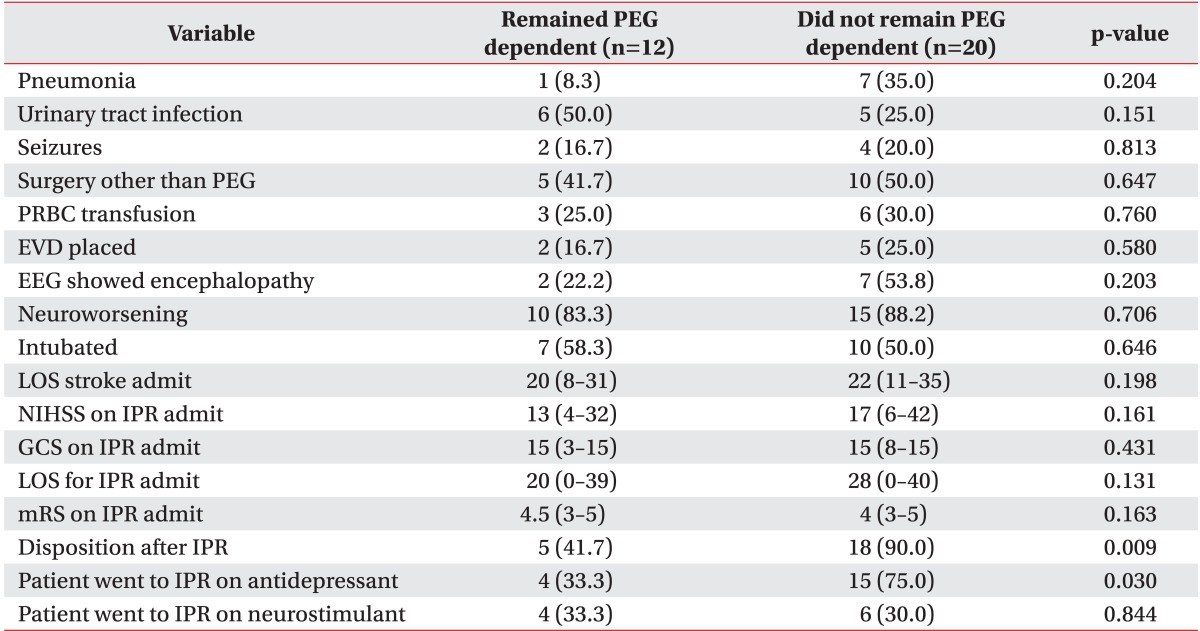
Values are presented as number (%) or median (range).
PEG, percutaneous endoscopic gastrostomy; PRBC, platelets red blood cells; EVD, external ventricular drainage; EEG, electrographic; LOS, length of stay; NIHSS, National Institute of Health Stroke Scale; IPR, inpatient rehabilitation; GCS, Glasgow Coma Scale; mRS, modified Rankin Scale.
Patients prescribed antidepressants at stroke discharge had a significantly higher swallow recovery percentage (75.0% vs. 33.3%, p=0.030) than those not treated. We found no significant difference in swallow recovery when comparing patients that used stimulants and those who did not use stimulants.
Although there was no statistically significant difference in IPR length of stay among patients who remained dependent and patients who regained swallowing (20 vs. 28 days, p=0.131), patients who recovered swallowing by discharge from IPR were more frequently discharged home compared to those who remained PEG-dependent (90.0% vs. 41.7%, p=0.009). The PEG patients who did not recover their swallow were discharged to skilled nursing facility (16.7%), discharged to a nursing home (25.0%), or expired (16.7%).
In the multivariable model controlling for age, left-sided strokes had a higher odds of recovering swallow function (Hosmer-Lemeshow goodness-of-fit p=0.2643; OR, 15.15; 95% CI, 1.32-173.34; p=0.028). We were not able to adjust for any other factors in the model due to the models failing to converge with additional covariates. We found no differences in the proportions of patients who completed at least one MBSS prior to PEG, had aspiration on MBSS or had incomplete assessments due to impaired mental status, on comparing the patients who recovered swallowing during IPR and those who remained PEG dependent.
DISCUSSION
We identified several potential predictors of early recovery of functional swallow, including younger age, left-sided stroke, and exposure to antidepressant medication. Our finding of younger age predicting swallow recovery is in keeping with another study (n=77) [6].
Our study suggests that patients with right-sided strokes are less likely to recover swallowing than left sided strokes. Swallowing musculature is represented on the cortices of both hemispheres but displays interhemispheric asymmetry, independent of handedness, with larger representation on the right hemisphere for volitional swallowing [3,13,14,15,16,17]. Following stroke, dysphagia has been reported to be associated with a smaller pharyngeal representation on the intact hemisphere [15]. This finding may be related to the larger representation of swallow function on the right hemisphere and greater risk of silent laryngeal penetration and aspiration with right-sided strokes [5,16,18]. Patients who were on antidepressants at discharge to IPR had a significantly higher swallow recovery percentage than those not treated. Antidepressant exposure has been associated with better outcomes in ischemic stroke patients (n=49-118) although these results are controversial [19,20,21]. Studies using transcranial magnetic stimulation found an increased excitability of the affected areas in the brain after intake of a single dose of a serotonergic drug by stroke patients [22]. The influence on excitability of the motor cortices presumably modulates brain plasticity and thereby the relearning of motor function after stroke. Improvement in dysphagia in our sample could be due to overall improvement of patient functionality, in other words antidepressants increased brain plasticity, that's why these patients are more capable of relearning how to swallow. In our study, patient functionality could not be assessed given lack of randomization of antidepressant administration and prospective standardized documentation of modified Rankin Scale after discharge from IPR.
In our study, hemorrhagic stroke was associated with significantly higher odds of having PEG placed. While hemorrhagic stroke tends to be more severe than ischemic stroke [23,24], we found no relationship of baseline or discharge NIHSS with recovery of functional swallow. Interestingly, hemorrhagic strokes were more likely to recover functional swallowing than ischemic strokes. While our results failed to reach statistical significance, the difference in swallowing recovery in intracranial hemorrhage patients relative to acute ischemic stroke patients is clinically significant. Even though a previous retrospective study (n=65) reported that neglect, facial weakness and dysarthria were more frequent in patients with less swallowing recovery [25], our results showed no association of these symptoms with remaining PEG dependent. We were unable to compare recovery of swallow based on infratentorial vs. supratentorial stroke location, due to very small numbers of patients who were discharged to IPR with PEG placement after infratentorial stroke.
Similar proportions of patients completed MBSS or had impaired mental status that prevented complete assessment of dysphagia in the two groups. Due to low numbers of patients, we were unable to demonstrate that aspiration on MBSS predicted persistent dependence on PEG feeds. The impaired level of consciousness prevented performance of a MBSS and would have prevented adequate caloric consumption had a functional swallow been documented. Presence of cortical involvement of stroke was not associated with better or worse swallowing recovery.
Patients who recovered swallowing were more likely to be discharged home from IPR than patients who remained PEG dependent. Discharge home after stroke is associated with tremendous lifetime cost savings (almost 20% direct costs) compared with chronic custodial care [26].
Our study is one of the few limiting the analyses to patients who are discharged to IPR after PEG was placed, during the admission for new stroke. A similar study [6] had a much lower discontinuation rate for PEG use after a substantially longer IPR stay. Since our overall PEG rate was lower than in the previous study, it is unlikely that our center has a lower threshold for PEG placement. Worse stroke severity could account for the difference in discontinuation rate, but NIHSS scores were not included in the earlier report. Younger age, as in our study, was found to be predictive of PEG removal prior to discharge from IPR.
Our study is not without limitations. It is limited by its small sample size and retrospective nature. Due to small sample size constraints we were limited in what we could adjust for in the final models. We decided on the variables in the final models based on model fit statistics and clinical relevance. Despite its limitations, our study is one of the few studies that have investigated factors predicting early PEG removal. Our study contributes additional evidence for some of the factors already identified, age and side of stroke. Further, we observed antidepressant use and hemorrhagic strokes, a previously unreported factor, to be predictors of early PEG removal. We recommend these predicting factors to be taken into consideration when deciding whether to place PEG or not, in patients with potential for rapid swallow recovery. Due to the exploratory nature of this manuscript we intend to investigate these findings in the future with more generalizable population and a prospective study to prove the utility of these predicting factors and the benefit of deferring PEG placement in patients who meet certain criteria.
Although PEG represents a safer and more effective intervention to manage long-term dysphagia [27], the efficacy of its use in the acute phase of stroke recovery (<3 weeks) is disputable [28]. Further research is needed to assess the clinically effective timing of PEG placements to support functional recovery of activity participation (including oral nutrition and hydration) among patients with acute stroke. Identification of predictors of swallow recovery could aid in setting expectations for duration of PEG when discussing PEG placement, determining which patients may be able to defer PEG placement and the risks and expenses that ensue by opting for continuation of NGT feeds while anticipating recovery of swallow function. Predictors of swallow recovery could also allow for negotiating with IPR medical directors to accept patients with short term modalities of enteral feeding (e.g., NGTs).
ACKNOWLEDGMENTS
This work was supported by a grant from the Doris Duke Charitable Foundation to fund Clinical Research Fellow Perry Dubin. The project described was supported by Awards (No. 5-T32-HS013852-10 from the Agency for Healthcare Research and Quality and No. 3-P60-MD000502-08S1 from the National Institute on Minority Health and Health Disparities, National Institutes of Health).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Smithard DG, O'Neill PA, England RE, Park CL, Wyatt R, Martin DF, et al. The natural history of dysphagia following a stroke. Dysphagia. 1997;12:188–193. doi: 10.1007/PL00009535. [DOI] [PubMed] [Google Scholar]
- 2.Barer DH. The natural history and functional consequences of dysphagia after hemispheric stroke. J Neurol Neurosurg Psychiatry. 1989;52:236–241. doi: 10.1136/jnnp.52.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar S, Langmore S, Goddeau RP, Jr, Alhazzani A, Selim M, Caplan LR, et al. Predictors of percutaneous endoscopic gastrostomy tube placement in patients with severe dysphagia from an acute-subacute hemispheric infarction. J Stroke Cerebrovasc Dis. 2012;21:114–120. doi: 10.1016/j.jstrokecerebrovasdis.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar S, Doughty C, Doros G, Selim M, Lahoti S, Gokhale S, et al. Recovery of swallowing after dysphagic stroke: an analysis of prognostic factors. J Stroke Cerebrovasc Dis. 2014;23:56–62. doi: 10.1016/j.jstrokecerebrovasdis.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Smithard DG, O'Neill PA, Martin DF, England R. Aspiration following stroke: is it related to the side of the stroke? Clin Rehabil. 1997;11:73–76. doi: 10.1177/026921559701100111. [DOI] [PubMed] [Google Scholar]
- 6.Ickenstein GW, Kelly PJ, Furie KL, Ambrosi D, Rallis N, Goldstein R, et al. Predictors of feeding gastrostomy tube removal in stroke patients with dysphagia. J Stroke Cerebrovasc Dis. 2003;12:169–174. doi: 10.1016/S1052-3057(03)00077-6. [DOI] [PubMed] [Google Scholar]
- 7.Geeganage C, Beavan J, Ellender S, Bath PM. Interventions for dysphagia and nutritional support in acute and subacute stroke. Cochrane Database Syst Rev. 2012;10:CD000323. doi: 10.1002/14651858.CD000323.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Gomes CA, Jr, Lustosa SA, Matos D, Andriolo RB, Waisberg DR, Waisberg J. Percutaneous endoscopic gastrostomy versus nasogastric tube feeding for adults with swallowing disturbances. Cochrane Database Syst Rev. 2010;(11):CD008096. doi: 10.1002/14651858.CD008096.pub2. [DOI] [PubMed] [Google Scholar]
- 9.James A, Kapur K, Hawthorne AB. Long-term outcome of percutaneous endoscopic gastrostomy feeding in patients with dysphagic stroke. Age Ageing. 1998;27:671–676. doi: 10.1093/ageing/27.6.671. [DOI] [PubMed] [Google Scholar]
- 10.Teasell R, Foley N, McRae M, Finestone H. Use of percutaneous gastrojejunostomy feeding tubes in the rehabilitation of stroke patients. Arch Phys Med Rehabil. 2001;82:1412–1415. doi: 10.1053/apmr.2001.25076. [DOI] [PubMed] [Google Scholar]
- 11.Savitskas RIu, Krishiunas AI. Dysphagia in patients with cerebral stroke during early inpatient rehabilitation. Zh Nevrol Psikhiatr Im S S Korsakova. 2006;(Suppl 17):62–65. [PubMed] [Google Scholar]
- 12.Bender R, Lange S. Adjusting for multiple testing: when and how? J Clin Epidemiol. 2001;54:343–349. doi: 10.1016/s0895-4356(00)00314-0. [DOI] [PubMed] [Google Scholar]
- 13.Martin RE, Sessle BJ. The role of the cerebral cortex in swallowing. Dysphagia. 1993;8:195–202. doi: 10.1007/BF01354538. [DOI] [PubMed] [Google Scholar]
- 14.Gordon C, Hewer RL, Wade DT. Dysphagia in acute stroke. Br Med J (Clin Res Ed) 1987;295:411–414. doi: 10.1136/bmj.295.6595.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamdy S, Aziz Q, Rothwell JC, Singh KD, Barlow J, Hughes DG, et al. The cortical topography of human swallowing musculature in health and disease. Nat Med. 1996;2:1217–1224. doi: 10.1038/nm1196-1217. [DOI] [PubMed] [Google Scholar]
- 16.Kern MK, Jaradeh S, Arndorfer RC, Shaker R. Cerebral cortical representation of reflexive and volitional swallowing in humans. Am J Physiol Gastrointest Liver Physiol. 2001;280:G354–G360. doi: 10.1152/ajpgi.2001.280.3.G354. [DOI] [PubMed] [Google Scholar]
- 17.Mann G, Hankey GJ, Cameron D. Swallowing disorders following acute stroke: prevalence and diagnostic accuracy. Cerebrovasc Dis. 2000;10:380–386. doi: 10.1159/000016094. [DOI] [PubMed] [Google Scholar]
- 18.Robbins J, Levine RL, Maser A, Rosenbek JC, Kempster GB. Swallowing after unilateral stroke of the cerebral cortex. Arch Phys Med Rehabil. 1993;74:1295–1300. doi: 10.1016/0003-9993(93)90082-l. [DOI] [PubMed] [Google Scholar]
- 19.Gainotti G, Antonucci G, Marra C, Paolucci S. Relation between depression after stroke, antidepressant therapy, and functional recovery. J Neurol Neurosurg Psychiatry. 2001;71:258–261. doi: 10.1136/jnnp.71.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dam M, Tonin P, De Boni A, Pizzolato G, Casson S, Ermani M, et al. Effects of fluoxetine and maprotiline on functional recovery in poststroke hemiplegic patients undergoing rehabilitation therapy. Stroke. 1996;27:1211–1214. doi: 10.1161/01.str.27.7.1211. [DOI] [PubMed] [Google Scholar]
- 21.Chollet F, Tardy J, Albucher JF, Thalamas C, Berard E, Lamy C, et al. Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. Lancet Neurol. 2011;10:123–130. doi: 10.1016/S1474-4422(10)70314-8. [DOI] [PubMed] [Google Scholar]
- 22.Robol E, Fiaschi A, Manganotti P. Effects of citalopram on the excitability of the human motor cortex: a paired magnetic stimulation study. J Neurol Sci. 2004;221:41–46. doi: 10.1016/j.jns.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS. Intracerebral hemorrhage versus infarction: stroke severity, risk factors, and prognosis. Ann Neurol. 1995;38:45–50. doi: 10.1002/ana.410380110. [DOI] [PubMed] [Google Scholar]
- 24.Andersen KK, Olsen TS, Dehlendorff C, Kammersgaard LP. Hemorrhagic and ischemic strokes compared: stroke severity, mortality, and risk factors. Stroke. 2009;40:2068–2072. doi: 10.1161/STROKEAHA.108.540112. [DOI] [PubMed] [Google Scholar]
- 25.Schroeder MF, Daniels SK, McClain M, Corey DM, Foundas AL. Clinical and cognitive predictors of swallowing recovery in stroke. J Rehabil Res Dev. 2006;43:301–310. doi: 10.1682/jrrd.2004.12.0154. [DOI] [PubMed] [Google Scholar]
- 26.Taylor TN, Davis PH, Torner JC, Holmes J, Meyer JW, Jacobson MF. Lifetime cost of stroke in the United States. Stroke. 1996;27:1459–1466. doi: 10.1161/01.str.27.9.1459. [DOI] [PubMed] [Google Scholar]
- 27.Rendall K, Pichard C, Louis SM, Reny JL. Enteral nutrition: nasogastric tube or percutaneous endoscopic gastrostomy? Rev Med Suisse. 2012;8:1972–1974. 1976–1977. [PubMed] [Google Scholar]
- 28.Dennis MS, Lewis SC, Warlow C FOOD Trial Collaboration. Effect of timing and method of enteral tube feeding for dysphagic stroke patients (FOOD): a multicentre randomised controlled trial. Lancet. 2005;365:764–772. doi: 10.1016/S0140-6736(05)17983-5. [DOI] [PubMed] [Google Scholar]


