Abstract
Injury to a target organ can be sensed by bone marrow stem cells that migrate to the site of damage, undergo differentiation, and promote structural and functional repair. This remarkable stem cell capacity prompted an investigation of the potential of mesenchymal and hematopoietic stem cells to cure acute renal failure. On the basis of the recent demonstration that hematopoietic stem cells (HSCs) can differentiate into renal cells, the current study tested the hypothesis that HSCs can contribute to the regeneration of renal tubular epithelial cells after renal injury. HSCs from human umbilical cord blood which isolated and purified by magnetic activated cell sorting were transplanted intraperitoneal into acute renal failure (ARF) rats which was established by a single dose of cisplatin 5 mg/kg for five days. The Study was carried on 48 male white albino rats, of average weight 120-150 gm. The animals were divided into 4 groups, Group one Served as control and received normal saline throughout the experiments. Group two (model control) received a single dose of cisplatin. Group three and four male-albino rats with induced ARF received interapritoneally (HSCs) at two week and four week respectively. Injection of a single dose of cisplatin resulted in a significant increase in serum creatinine and urea levels, histo-pathological examination of kidney tissue from cisplatin showed severe nephrotoxicity in which 50-75% of glomeruli and renal tubules exhibited massive degenerative change. Four weeks after HSC transplantation, Serum creatinine and urea nitrogen decreased 3.5 times and 2.1 times as well as HGF, IGF-1, VEGF and P53 using quantitative real-time PCR increased 4.3 times, 3.2, 2.4 and 4.2 times compared to ARF groups, respectively. The proliferation of cell nuclear antigen (PCNA)-positive cells (500.083±35.167) was higher than that in the cisplatin groups (58.612±15.743). In addition, the transplanted umbilical cord hematopoietic stem cells UC-HSCs could reside in local injury sites, leading to the relief of hyperemia and inflammation, but no obvious transdifferentiation into renal-like cells. The results lay the foundation for further study on the potential application of UC-HSCs in human disease and Because of their availability; HSC may be useful for cell replacement therapy of acute renal failure.
Keywords: Acute kidney injury, umbilical cord hematopoietic stem cells, cisplatin, nephron repair, stem cells
Introduction
Acute kidney injury (AKI) is found in 5% of all hospitalized patients and in up to 50% of patients with sepsis [1,2]. With a mortality of up to 60% in patients on the intensive care unit, AKI thus provides one of the big challenges in modern acute care nephrology. Furthermore, it is becoming increasingly evident that not only renal failure necessitating replacement therapy is associated with poor outcome but that even a small rise in serum creatinine confers a marked mid- or long-term risk of death or for the development of end-stage renal disease [3]. To date, no effective therapies are at hand to prevent or treat AKI specifically. In the last 10 years, the mounting knowledge of the plasticity of adult stem cells raised the hope for a new and potent therapeutic approach in this respect. Early investigations using chimeric mice have demonstrated that bone marrow-derived stem cells (BMSCs) home to the injured kidney and become integrated into the tubular epithelium, the predominant site of injury in AKI [4].
The majority of these studies, focused on the use of bone marrow-derived multi-potent stromal cells, also referred to as mesenchymal stem cells (MSCs). Although a beneficial impact of MSCs on kidney function in various AKI animal models has been clearly shown by various groups, this is apparently not caused by direct tubular incorporation but rather paracrine or endocrine effects [5-7]. Much less is known about the effect of hematopoietic stem cells (HSCs), the other major fraction of the BMSC, in AKI.
On the other hand, therapeutic application of HSCs certainly poses a big challenge to clinical use. However, since early in ontogenetic development haematopoiesis arises in the aorta-gonad mesonephros region, which is also the origin of the later kidney [8], it is the HSCs that were originally considered especially useful for kidney repair. In addition, HSCs are known to 3
within the blood and immune system, as well as cells of non-hematopoietic tissues, such as hepatocytes, cardiac myocytes, gastrointestinal epithelial cells, and vascular endothelial cells [14-18]. The discovery that adult HSC can cross lineage boundaries to become cells of other tissues has challenged the traditional view that somatic stem cells are lineage-restricted and organ-specific [19]. One Possibility is that HSC retain developmental plasticity and can be reprogrammed to express genes that are required to differentiate into the cells of the organs into which they are introduced. Another distinguishing feature of HSC is their ready availability from bone marrow, cord blood, and mobilized peripheral blood. This property makes HSC potentially useful for cell replacement therapy in regenerative medicine.
The S3 segments of the proximal tubules located in the outer stripe of the outer medulla are particularly susceptible to ischemic injury and are primarily responsible for the pathophysiological and clinical presentations of ARF [20-22]. Recovery from ARF requires the replacement or regeneration of lost tubular epithelial cells. This process is accompanied by complex changes in gene expression of growth modulatory molecules, such as EGF, IGF-1, and HGF [23,24]. It has been generally believed that some of the surviving renal tubular cells dedifferentiate and reenter the cell cycle to produce epithelial cells that rebuild the structure and function of the renal tubules.
Cisplatin (cis-diamminedichloroplatinum (II), CDDP) is an antineoplastic drug used in the treatment of many solid-organ cancers, including those of the head, neck, lung, testis, ovary, and breast [25-28].
There are several mechanisms that contribute to renal dysfunction following exposure to cisplatin that include direct tubular toxicity in the form of apoptosis and necrosis that is mediated through inflammation, reactive oxygen species (ROS), calcium overload, phospholipase activation, depletion of reduced glutathione, inhibition of mitochondrial respiratory chain function, induction of apoptosis, opening of mitochondrial permeability transition pore (MPTP) and ATP depletion [29-32].
In these studies we show that mobilized human umbilical cord blood CD34+ stem/progenitor cells are recruited to the injured kidney and promote survival, vascular regeneration and functional recovery.
Materials and methods
Chemical agents
Cisplatin (cis-diamminedichloroplatinum (II), CDDP) was obtained from sigma-Aldrich (Germany). Provided as a powder dissolved in saline (1 mg/ml). Cisplatin (Trade name-cisplatin, Brand name-Platin, is a chemotherapeutic drug. It was a first member of class, platinum containing anticancer drug, which now also include carboplatin and oxaliplatin. The platinum complex react in vivo, binding to and causing cross linking of DNA, which ultimately triggers apoptosis (Programmed cell death).
Preparation of the animal model
Experimental animals: The study was carried on 48 male white albino rats, of an average weight 150-200 gm. Rats were bred and maintained in an air-conditioned animal house with specific pathogen free conditions, and were subjected to a 12:12-h daylight/darkness and allowed unlimited access to chow and water. The animals were housed in plastic cages. Each experimental group consisted of 12 animals for each treatment and control. All the ethical protocols for animal treatment were followed and supervised by the animal facilities, Faculty of Women’s, Ain Shams University. They were divided into 4 groups as follow:
Group 1: received normal saline (5 mg/kg), injected i. p and served as control group (n=12).
Group 2: received a single dose of cisplatin (5 mg/kg) and served as a model group (n=12) (the animals will be injected with cisplatin to induce acute renal failure. At the fifth day, all animals were anaesthetized with ether; blood samples were taken out from retro-orbital sinus of rat.
Group 3: 12 male albino rats with induced acute renal failure will received an intraperotineal injection of umbilical cord hematopoietic stem cells, in a dose of 3x (106) 24 hours after the induction of acute renal failure. The study group (renal injury with injection of hematopoietic stem cells) will be sacrificed after 2 weeks.
Group 4: It is consisted of 12 albino male rats with induced acute renal failure received an intraperotineal injection of umbilical cord hematopoietic stem cells, in a dose of 3x (106) 24 hours after the induction of acute renal failure. At the planned time 4 weeks after HSCs injection rats were scarified.
Blood samples were collected from the retro-orbital vein. Sera were separated and used for measurement of Creatinine and urea.
The kidney tissues were immediately removed and were examined for:
Quantitative analysis of hepatocyte growth factor gene (HGF), insulin-like growth factor gene (IGF-1), vascular endothelial growth factor gene (VEGF) for neovascularization and P53 gene expression by quantitative real time qPCR.
Histopathological examination of renal tissue by haematoxylin and eosin.
Immunohistochemistry also detected using PCNA antibody.
Detection of the stem cells homing in kidney tissue after its labeling with PKH26 dye by fluorescent microscope to detect its red fluorescence.
Preparation, isolation and identification of HSCs in culture
a- Cell Source: Human Umbilical Cord Blood (UCB) was used for Separation of mononuclear cells (MNCs) after obtaining an informed consent and research ethics committee approval.
b- Cell Isolation:
Preparation of cord blood cells:
• Anticoagulated cord blood was diluted 1:4 with PBS containing 2 mM EDTA (Gibco-Invitrogen, Grand Island, NY) and 35 ml of the diluted sample was carefully layered on 15 ml Ficoll-Paque (Gibco-Invitrogen, Grand Island, NY), then they were centrifuged for 35 min. at 400xg rpm.
• The upper layer was aspirated leaving the MNC layer undisturbed at the interphase.
• The interphase layer (MNC layer) was carefully aspirated and Washed twice in PBS containing 2 mM EDTA and centrifuged for 10 minutes at 200xg rpm at 20°C.
• The cell pellet was resuspended in a final volume of 300 μl of buffer.
Magnetic labeling of CD34+ cells using MACS (magnetic cell sorting) kits (MiniMACS; Miltenyi Biotec, Bergisch Gladbach, Germany):
Components:
• MACS colloidal super-paramagnetic Multisort Microbeads conjugated to monoclonal mouse anti-human CD34+ antibody.
• Fc-receptor (FcR) blocking reagent contains human Ig.
Magnetic labeling of cells in suspension:
• 100 μl of FcR blocking reagent was added to the cell suspension to inhibit unspecific or Fc-receptor binding of CD34+ Multisort Microbeads to non-target cells.
• Cells were labeled by adding 100 μl CD34+ Multisort Microbeads per 108 total cells. And were incubated for 30 min. in the refrigerator at 6°C-12°C.
• After incubation, cells were washed carefully and resuspended in 300 μl buffer.
Magnetic separation of CD34+ cells using MACS
• A positive selection column was placed in the Mini-MACS Separator and was washed with appropriate amount of buffer.
• Cell suspension was applied onto the column and negative cells were allowed to pass through.
• The column was removed from the separator, appropriate amount of the buffer was added and CD34+ cells were collected by flushing out the column using the plunger supplied with the column.
Isolation of CD34+ cells was confirmed by Florescent Analysis Cell Sorting (FACS) at clinical pathology department, Kasr Al-Einy.
Labeling stem cells with PKH26 dye
CD34+ cells were harvested during the 4th passage and were labeled withPKH26 fluorescent linker dye (Sigma, Aldrich, and Saint Louis, USA). PKH26 is a red fluorochrome, has excitation (551 nm) and emission (567 nm). The linkers are physiologically stable and show little to no toxic side-effects on cell systems. Labeled cells retain both biological and Proliferating activity, and are ideal for in vitro cell labeling, in vitro Proliferation studies and long term, in vivo cell tracking. Labeled cells that have been washed can be visualized in culture up to 100 days after staining (for non-dividing cells). This enhanced stability is favorable for long term in vivo studies. The dye itself is stable and will divide equally when the cells divide. After staining with PKH dyes, one can observe as many as 8 divisions depending on how brightly the cells were stained initially and the amount of surface area on the cells. Most commonly, 4-6 divisions can be visualized.
Real-time quantitative analyses for VEGF, HGF and IGF-1 gene expression
Total RNA was extracted from kidney tissue homogenate using RNeasy purification reagent (Qiagen, Valencia, CA). CDNA was generated from 5 μl of total RNA extracted with 1 μl (20 pmol) antisense primer and 0.8 μl superscript AMV rever transcriptase for 60 min at 37°C.
The relative abundance of mRNA species was assessed using the SYBR® Green method on an ABI prism 7500 sequence detector system (Applied Biosystems, Foster City, CA). PCR primers were designed with Gene Runner Software (Hasting Software, Inc., Hasting, NY) from RNA sequences from GenBank (Table 1). All primer sets had a calculated annealing temperature of 60°. Quantitative RT-PCR was performed in duplicate in a 25-μl reaction volume consisting of 2X SYBR Green PCR Master Mix (Applied Biosystems), 900 nM of each primer and 2-3 μl of cDNA. Amplification conditions were 2 min at 50°, 10 min at 95° and 40 cycles of denaturation for 15 s and annealing/extension at 60° for 10 min. Data from real-time assays were calculated using the v1·7 Sequence Detection Software from PE Biosystems (Foster City, CA). Relative expression of VEGF, HGF and IGF-1 mRNA was calculated using the comparative Ct method. All values were normalized to the beta actins genes and reported as fold change over background levels detected in ARF.
Table 1.
Sequence of the Primers Used for Real-Time PCR
| Primer sequence | |
|---|---|
| 1- HGF | Forward: 5’-TCACACAGAATCAGGCAAGACT-3’; |
| Reverse: 3’-AAGGGGTGTCAGGGTCAA-5’. | |
| 2- INF-1 | Forward : 5’-AGGCTATGGCTCCAGCATTC-3’; |
| Reverse: 3’-AGTCTTGGGCATGTCAGTGTC-5’. | |
| 4- VEGF | Forward: 5’-ACCCCGACGAGATAGAGTACAT-3’; |
| Reverse: 3’-CTTCTAATGCCCCTCCTTGT-5’. | |
| 5- P53 | Forward: 5’-GCCTGAAATCTACCAGATCATGTTG-3’; |
| Reverse: 3’-TTCCACAAGCTCCACGAATCTT-5’. | |
| 6- Beta actins | Forward: 5’-TGTTGTCCCTGTATGCCTCT-3’; |
| Reverse: 3’-TAATGTCACGCACGATTTCC-5’. |
Biochemical analysis
Serum urea and creatinine levels were measured using the conventional colorimetric method using Quanti Chrom TM assay kits based on the improved Jung and Jaffe methods, respectively (DIUR-500 and DICT-500).
Histological examinations
Animals from experimental group were sacrificed immediately after 15 and 30 d of HSCs transplantation and kidney was quickly removed and fixed in 10% neutral formalin. Fixed materials were embedded in paraffin wax and sections of 5-μm thickness were cut. Slides were stained with hematoxylin and eosin (H & E) for histological examination.
Immunohistochemistry assay
Proliferating cell nuclear antigen (PCNA) is an auxiliary protein of DNA polymerase δ to play a fundamental role in the initiation of cell proliferation, and it is expressed strongly in the nucleus during late G1 phase immediately before the onset of DNA synthesis, becoming maximal during S phases and declining during G2 and M phase. Its level correlates directly with rates of cellular proliferation and DNA synthesis.
To determine the number of proliferating tubular cells, the expression of proliferating cell nuclear antigen (PCNA) was detected by immunohistochemistry. Section (5 Mm) were deparaffinized with xylene and rehydrated with ethanol. The sections were subjected to an antigen retrieval procedure according to the manufacturer’s instruction (thermo fisher scientific, Fremont, CA94538, USA). in brief; Na-citrate buffer (10 mmol/L, pH 6.5) was preheated in the microwave. The sections were then soaked in the solution and cooked for 15 minutes in the microwave, then cool down at room temperature for 20 minutes. To reduce nonspecific background staining due to endogenous peroxidase, incubate slide in hydrogen peroxide for 10-15 minutes. Apply Ultra V Block and incubate for 5 minutes at room temperature to block nonspecific background staining. Apply primary antibodies Two to three drops of PCNA antibody incubate according to manufacturer’s protocol.
Two to three drops of secondary antibody (biotinylated polyvalent) were placed on each slide. The slides were incubated for 15 min. at room temperature in a humid chamber. Apply Streptavidin Peroxidase and incubate for 10 minutes at room temperature. Incubate with peroxidase-compatible chromagen of choice according to manufacturer’s recommendations. Counter stain was done then the slides were washed in tap water.
Quantification of immunostaining
Each tissue sample stained for PCNA was viewed and scored by an observer blinded to the treatment received. Quantitation of tubular cells was performed with binocular microscope on 10 fields outside the glomeruli area. The mean of10 fields were taken and entered in statistical analysis.
Statistical analysis
Data were coded and entered using the statistical package SPSS version 21. Data was summarized using mean and standard deviation for quantitative variables. Comparisons between groups were done using analysis of variance (ANOVA) with multiple comparisons post hoc test. P-values less than 0.05 were considered as statistically significant. The arithmetic mean (M): The mean is the sum of the observations divided by the number of observations:
M=s (x)/n
S (x)=sum of individual values.
n=number of measurements.
Standard Deviation (SD) (Equation 1):
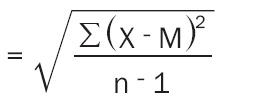 |
n-1=degree freedom (34)
Results
Results of the in vitro study
Identification of CD34+ cells by fluorescence-activated cell sorting (FACs) (Figure 1). CD34+ labeled with PKH26 fluorescent dye was detected in the renal tissues confirming that these cells homed into the kidney tissue (Figure 2).
Figure 1.
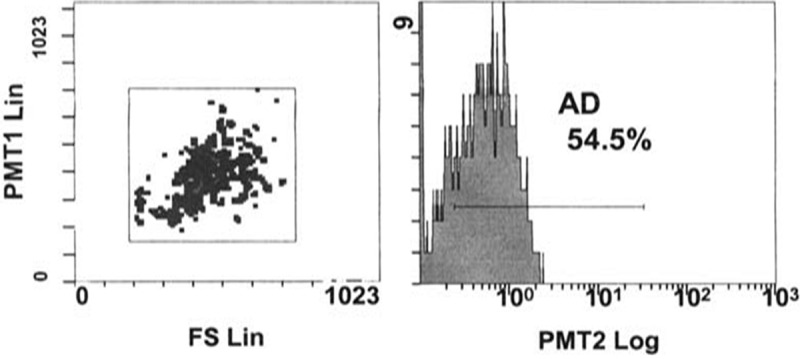
FACS analysis of cells revealed 54.5% positive for CD34.
Figure 2.
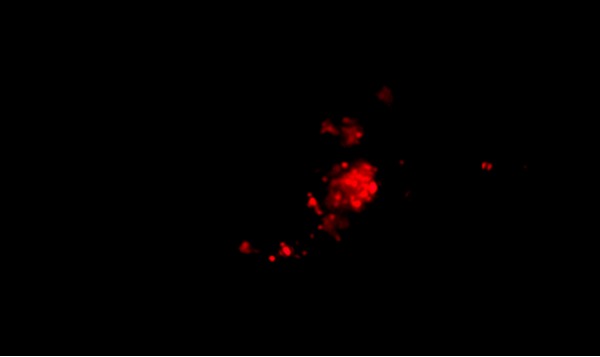
PKH26 staining of cells in humanized rat kidney.
Results of the in vivo studies
Detection of homing of the injected human cells by detection of PKH-26 fluorescent dyes in rat kidney:
Cells labeled with the PKH-26 fluorescent dye showed strong red auto fluoresce after transplantation in rats, confirming that these cells were actually seeded into the renal tissue Figure 2.
Biochemical analysis of kidney functions:
• There was a significant increase (p < 0.05) in the mean urea and creatinine levels in the acute renal failure group (84.43±10.02), (1.18±0.41) respectively compared to the control group (34.85±8.19), (0.10±0.05).
• There was a significant decrease (p < 0.05) in the mean urea and creatinine levels in the group receiving CD34 for 2 w (61.78±11.33), (0.53±0.31) compared to the acute renal failure group (84.43±10.02), (1.18±0.41) and there was a significant increase (p < 0.05) in the mean urea and creatinine levels in the group receiving CD34 for 2 w (61.78±11.33), (0.53±0.31) compared to the control group (34.85±8.19), (0.10±0.05) respectively.
• There was a significant decrease (p < 0.05) in the mean urea and creatinine levels in the group receiving CD34 for 4 w (55.03±11.58), (0.40±0.20) respectively compared to the acute renal failure group (84.43±10.02), (1.18±0.41). and there was a significant increase (p < 0.05) in the mean urea and creatinine levels in the group receiving CD34 for 4 w (55.03±11.58), (0.40±0.20) respectively compared to the control group (34.85±8.19), (0.10±0.05) (Table 2).
Table 2.
Mean of Serum Urea, Creatinine, K and Na level in Different Studied Groups
| Groups | Mean±SD (mg/dl) urea | Mean±SD (mg/dl) creatinine | Mean±SD (mg/dl) K | Mean±SD (mg/dl) Na |
|---|---|---|---|---|
| 1- Control | 34.85±8.19 | 0.10±0.05 | 4.86±0.30 | 149.50±4.95 |
| 2- Acute renal failure (ARF) | 84.43±10.02* | 1.18±0.41* | 2.73±0.49* | 127.38±2.61* |
| 3- ARF+CD34 (for 2 w) | 61.78±11.33*,# | 0.53±0.31# | 3.26±0.51* | 133.98±3.75*,# |
| 4- ARF+CD34 (for 4 w) | 55.03±11.58*,# | 0.40±0.20# | 4.02±0.32*,#,$ | 137.30±3.82*,# |
Values are represented as mean±SD.
statistically significant compared to corresponding value in control group (P < 0.05).
statistically significant compared to corresponding value in positive control group (P < 0.05).
statistically significant compared to corresponding value in CD34 2 week group (P < 0.05).
• There was a significant decrease (p < 0.05) in the mean Na, K levels in the acute renal failure group (127.38±2.61), (2.73±0.49), compared to the control group (149.50±4.95), (4.86±0.30).
• There was a significant decrease (p < 0.05) in the mean Na, K levels in the acute renal failure group receiving CD34 for 2 w (133.98±3.75), (3.26±0.51), compared to the control group (149.50±4.95), (4.86±0.30). and there was a significant increase (p < 0.05) in the mean Na levels in the acute renal failure group receiving CD34 for 2 w (133.98±3.75), compared to the acute renal failure group (127.38±2.61). There was a significant increase (p < 0.05) in the mean Na, k levels in the acute renal failure group receiving CD34 for 4 w (137.30±3.82), (4.02±0.32), compared to the acute renal failure group (127.38±2.61) more over K levels in the acute renal failure group receiving CD34 for 4 w (4.02±0.32) was a significant increase (p < 0.05), compared to the acute renal failure group receiving CD34 for 2 w (3.26±0.51) (Table 2).
QRT PCR for HGF, INF-1, VEGF and P53 in kidney tissue
Concerning gene expression, HGF, IGF-1, VEGF and P53 genes were significantly decreased in ARF group (P < 0.05) (2.20±1.15), (0.20±0.09), (1.09±0.18) and (0.26±0.23) respectively compared to control group (11.75±1.37) for HGF, (0.87±0.12) for IGF-1, (3.87±0.91) for VEGF and (1.58±0.36) for P53 (Table 3 and Figure 3).
Table 3.
Mean of Relative Gene Expression of HGF, IGF-1, VEGF and P53 in Kidney Tissue in all Studied Groups
| Groups | Mean±SD HGF | Mean±SD IGF-1 | Mean±SD VEGF | Mean±SD P53 |
|---|---|---|---|---|
| 1- Control | 11.75±1.37 | 0.87±0.12 | 3.87±0.91 | 1.58±0.36 |
| 2- Acute renal failure (ARF) | 2.20±1.15* | 0.20±0.09* | 1.09±0.18* | 0.26±0.23* |
| 3- ARF+CD34 (for 2 w) | 7.49±1.33*,# | 0.31±0.04* | 2.18±0.47*,# | 0.72±0.12*,# |
| 4- ARF+CD34 (for 4 w) | 8.27±1.28*,# | 0.59±0.11*,#,$ | 2.46±0.50*,# | 1.09±0.14*,# |
statistically significant compared to corresponding value in control group (I) (P < 0.05).
statistically significant compared to corresponding value in positive control group (ARF) (II) (P < 0.05).
statistically significant compared to corresponding value in CD34 2 week group (III) (P < 0.05).
Figure 3.
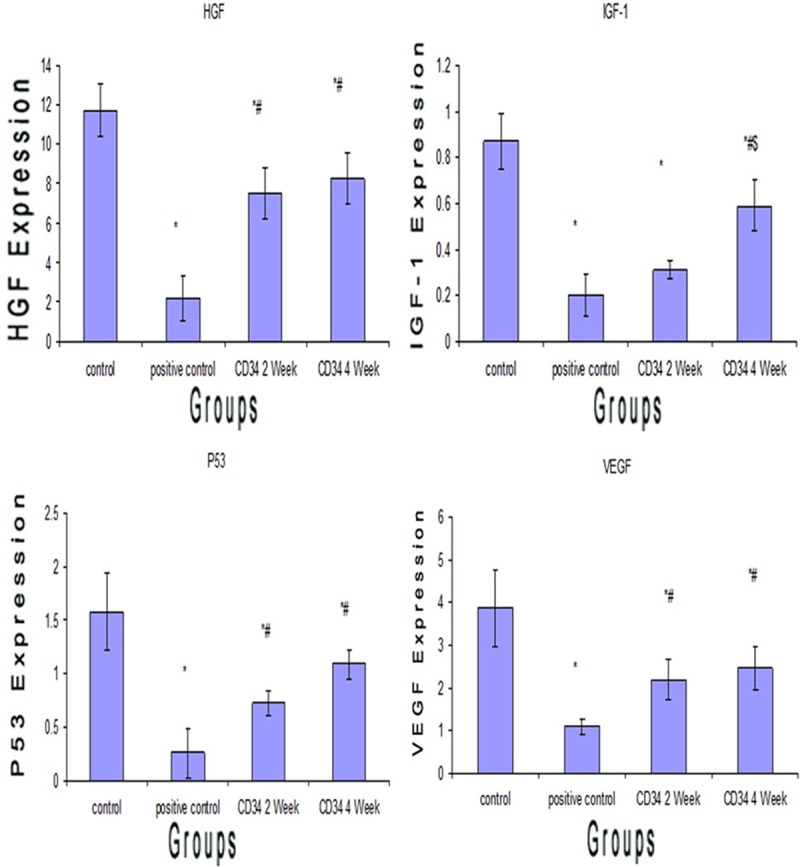
Histogram of the HGF, IGF-1 VEGF, and P53 gene expressions in Rat Kidney Tissue of Studied Groups. Values are represented as mean±SD. *: statistically significant compared to corresponding value in control group (I) (P < 0.05). #: statistically significant compared to corresponding value in positive control group (ARF) (II) (P < 0.05). $: statistically significant compared to corresponding value in CD34 2 week group (III) (P < 0.05).
• The mean HGF level in the acute renal failure group receiving CD34 for 2 w (7.49±1.33) showed a significant increase (p < 0.05) when compared to the acute renal failure group (2.20±1.15). Also The mean VEGF, P53 level in the acute renal failure group receiving CD34 for 2 w (2.18±0.47), (0.72±0.12) showed a significant increase (p < 0.05) when compared to the acute renal failure group (1.09±0.18), (0.26-±0.23). Moreover, HGF, IGF-1, VEGF and P53 genes were significantly decreased in ARF group receiving CD34 for 2 w (P < 0.05) compared to the control group (Table 3).
• The mean HGF, IGF-1, VEGF and P53 level in the acute renal failure group receiving CD34 for 4 w (8.27±1.28), (0.59±0.11), (2.46±0.50) and (1.09±0.14) showed a significant increase (p < 0.05) when compared to the acute renal failure group (2.20±1.15), (0.20±0.09), (1.09±0.18) and (0.26±0.23). Also the mean IGF-1 level in the acute renal failure group receiving CD34 for 4 w (0.59±0.11) showed a significant increase (p < 0.05) when compared to the acute renal failure group receiving CD34 for 2 w (0.31-±0.04). Moreover, HGF, IGF-1, VEGF and P53 genes were significantly decreased in ARF group receiving CD34 for 4 w (P < 0.05) compared to the control group (Table 3 and Figure 3).
Immunohistochemistry analysis of kidney tissue of different groups
Distribution of PCNA immunoreactivity (Table 4 and Figure 5). Five days after cisplatin administration, the number of positive cells was significantly higher in renal tissue of animals treated by cisplatin versus control group. PCNA stained cells were also significantly higher in group receiving CD34 for 4 w than cisplatin alone and control group.
Table 4.
Numbers of tubular cell nuclei stained with proliferating cell nuclear antigen (PCNA) in the cortical and cortico-medullary areas in Studied Groups
| Groups | Mean±SD PCNA |
|---|---|
| 1- Control | 5.100±2.108 |
| 2- Acute renal failure (ARF) | 58.612±15.743* |
| 3- ARF+CD34 (for 2 w) | 411.622±23.176*,# |
| 4- ARF+CD34 (for 4 w) | 500.083±35.167*,#,$ |
statistically significant compared to corresponding value in control group (P < 0.05).
statistically significant compared to corresponding value in positive control group (P < 0.05).
statistically significant compared to corresponding value in CD34 2 week group (P < 0.05).
Figure 5.
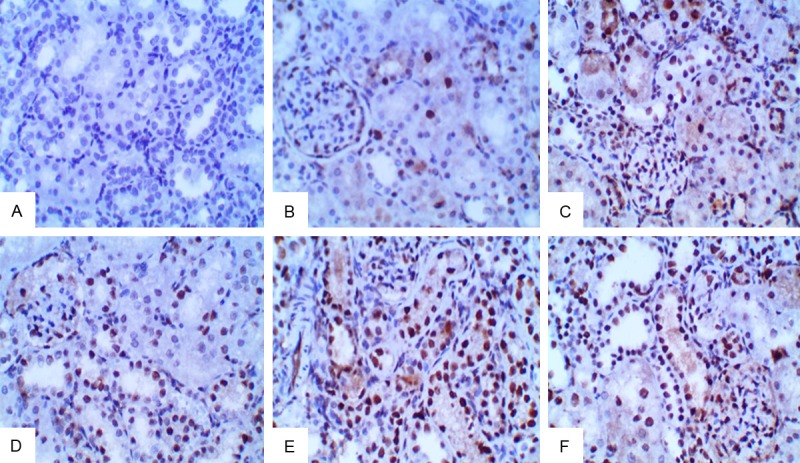
High magnification (PCNA x400). PCNA staining for normal control kidney treated with saline (A) PCNA x400; PCNA staining for group treated with cisplatin at five days showed some positive PCNA immuno-reactive nuclei in the lining tubular epithelial cells (B) PCNA x400; PCNA staining showed many positive PCNA immuno-reactive nuclei in the lining tubular epithelial cells (C, D) for (2 W) PCNA x400 and (E, F) for (4 W) showed apparent increase in positive PCNA immuno-reactive nuclei in comparison to other groups PCNA x400.
There was a significant increase (p < 0.05) in the mean PCNA proliferations in the acute renal failure group (58.612±15.743) compared to the control group (5.100±2.108) (Figure 4).
Figure 4.
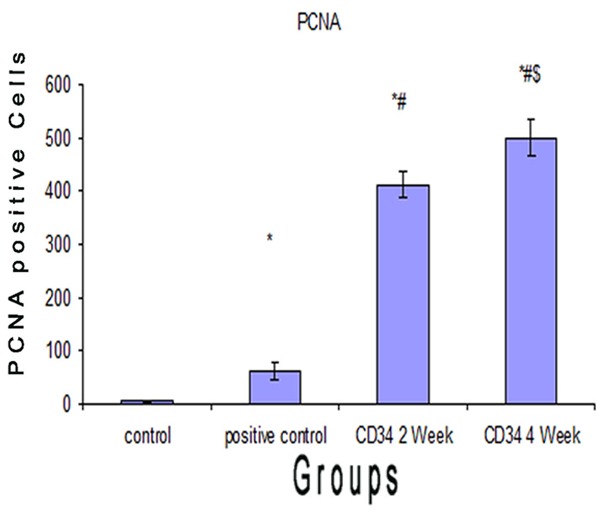
Comparison between the PCNA proliferations in rat kidney tissue of studied groups. Values are represented as mean±SD. *: statistically significant compared to corresponding value in control group (P < 0.05). #: statistically significant compared to corresponding value in positive control group (P < 0.05). $: statistically significant compared to corresponding value in CD34 2 week group (P < 0.05).
There was a significant increase (p < 0.05) in the mean PCNA proliferations in the group receiving CD34+ for 2 w (411.622±23.176) compared to the control group (5.100±2.108) and acute renal failure group (58.612±15.743).also There was a significant increase (p < 0.05) in the mean PCNA proliferations in the group receiving CD34+ for 4 w (500.083±35.167) compared to the control group (5.100±2.108), acute renal failure group (58.612±15.743) and the group receiving CD34+ for 2 w (411.622±23.176) (Table 4 and Figure 4). These results indicate that umbilical cord CD34+ promote regeneration and exert an anti-apoptotic effect in cisplatin-induced nephrotoxicity (Figure 5).
Histopathological changes
Examination of sections in the renal cortex and medulla of control groups showed no differences (Figure 6A, 6B; x400, x200 respectively). Histopathological examination of kidney tissue of ARF group showed Tubular atrophy of both proximal & distal tubules with marked lumen dilatation & cell debris in lumen & patchy loss of proximal tubule cells with pyknotic nuclei and regenerative change in tubular cells. (Figure 6C, 6D; x400), following CD34+ injection for 2 w there was marked distortion of renal corpuscles with partial destruction of glomeruli and thickening of parietal layer of Bowman’s capsule. Interstitial nephritis and cellular debris in Lumina of tubules are noted. Also some dividing cell are showed (Figure 6E, 6F; x400). In ARF/CD34+ for 4 w there was normal histological architecture of renal corpuscle and tubules. Moreover, Dividing cells are noted within the lining tubular epithelium (Figure 6G-I; x400).
Figure 6.
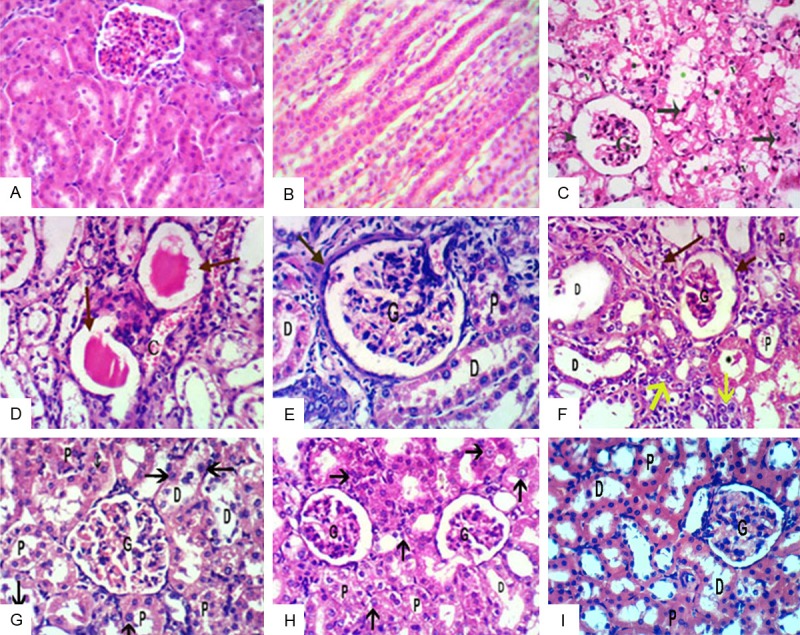
Histological changes from the control, cisplatin-treated rats and cisplatin administered umbilical cord derived CD34+ cells (UC-HSCs), respectively, five days after the cisplatin injection. (A, B: H & E x400, H & E x200 respectively) Representative hematoxylin and eosin staining of kidney sections from the control showed no differences in the renal cortex and medulla. (C, D) Kidney of a cisplatin-treated rat showing distorted glomerular capillary tufts (G) and widening of Bowman’s space (arrowhead) of Malpighian renal corpuscle. The lining tubular epithelial cells show marked cytoplasmic vacuolation (v), pyknotic nuclei (black arrows) and cellular debris in Lumina (black stars). Widened tubular lumen is noted (green star). Dense hyaline cast, peritubular congestion (c) in D (H & E x400). (E: H & E x400) cisplatin+CD34+ for 2 w group showing marked distortion of renal corpuscles with partial destruction of glomeruli (G) and thickening of parietal layer of Bowman’s capsule (black arrows). (F: H & E x400) showing interstitial nephritis (black arrows). cellular debris in Lumina of tubules (black stars). Partial destruction of glomerular tuft (G) and widened bowman’s space (arrowhead). some dividing cells are noted (yellow arrows). Finally cisplatin+CD34+ for 4 w group (G-I) H & E x400 showing improvement in renal tissue and normal histological architecture of renal corpuscle and tubules. Dividing cells are noted within the lining tubular epithelium (black arrows in G, H).
Discussion
In the lack of effective therapies, stem cell technology has recently emerged as a new and promising treatment option for AKI. With their plasticity and capability to trans-differentiate into cells of various tissue types, stem cells derived from the bone marrow and umbilical cord blood have been extensively studied with respect to their potential to enhance recovery of the injured tubular epithelium. For some time MSCs have been considered the major fraction of BMSCs responsible for this phenomenon and, indeed, therapeutic injection of MSCs after induction of renal injury leads to a significant attenuation of AKI, both, in functional and histomorphologic terms [33,34]. However, subsequently, it has been demonstrated that MSCs do not engraft directly into the tubular epithelium, and it is now accepted that paracrine or endocrine actions convey the reported beneficial effects. In turn, this makes HSCs the prime bone marrow stem cells (BMSC) fraction responsible for the cell replacement effect seen with whole bone marrow transplantation (BMT).
The result of the present work revealed that a single injection of cisplatin dose 5 mg/kg body weight in rat caused increase in serum creatinine level, blood urea nitrogen indicating induction of acute renal failure. Our results were similar to those of [35], who proved that cisplatin, have multiple intracellular effects, such as direct toxicity with reactive oxygen species, by activating mitogen activated protein kinases (MAPKinase), by inducing apoptosis, and by stimulating inflammation and fibrogenesis.
That result is supported by [36] who suggest that cisplatin may additionally directly induce necrosis and apoptosis of renal tubular cells. The toxic mechanisms have been suggested to imply the p53-mediated activations of caspases-2, 8 and 3 in cisplatin induced renal cell apoptosis, while oxidative stress-induced TNF-alpha synthesis via p38 MAPK phosphorylation may be the corner stone for renal tubular cells necrosis. Also, [32] supported the same conclusion, provided that Cisplatin is a platinum co-ordination complex that is hydrolyzed intracellularly to produce a high reactive moiety which cause cross linking of DNA.
The impact of injected exogenous HSCs in AKI has not yet been deciphered consistently. Initial studies using a model of cisplatin-induced AKI did not show significant beneficial effects [33]. In contrast, in a murine BMT model, Lin et al., [37] analyzed the contribution of HSCs in tubular regeneration after ischemia/reperfusion injury (I/R). Isolated HSCs of Rosa29 mice were injected into irradiated recipients that had been subjected to I/R. Using several detection methods, donor HSCs were identified in the S3 segment of the proximal tubule. However, this was 4 weeks after I/R and the short-term effect of HSC transplantation in terms of structural and, more importantly, functional restoration of the kidney was not examined in this investigation. Recently, [38] robustly repopulated bone marrow after lethal irradiation by transplanting plastic non-adherent enhanced green fluorescent-positive marrow cells as a source of hematopoietic lineage-committed bone marrow cells (HLMCs) and plastic-adherent MSCs in mice. After induction of AKI by administration of HgCL2, only HLMCs but not MSCs were found to be incorporated into the tubular epithelium. Together, these latter studies suggest that intrinsic HSCs may indeed play an important role in tubular turnover as well as in tubular regeneration after AKI [39].
In order to close this gap, we administered umbilical cord blood CD34+ cells, after throw determining their HSC properties, by intraperitoneal injection directly after inducing AKI in rats. HSC injections attenuate the extent of renal failure as mirrored by peak serum creatinine levels and it accelerates functional recovery. Moreover, histomorphologic features of AKI were also influenced by HSC application. our result were similar to those of [40] who proved that, hematopoietic stem and progenitor cells can be converted into renal-like cells ex vivo by sequentially treating them with a combination of protein factors and that these cells ameliorate AKI in a mouse model of I/R .
The results of this study demonstrate that transplanted HSC can contribute to renal tubular regeneration after I/R injury. Conventionally, it has been thought that injured tissues are repaired by proliferation of surviving parenchymal cells [41]. An increasing body of evidence has revealed that somatic stem cells can be mobilized from one organ to a different organ where they can differentiate into the cells of the recipient organ and participate in structural and functional regeneration [42,43]. The term “transdifferentiation” has been suggested to describe the phenotypic conversion of pluripotent somatic stem cells of one tissue type to another tissue type [41].
Fusion or transdifferentiation, this could not be answered in this study; however, both techniques definitely proved that those cells were able to maintain high population all through the study. These results agree with those of [44] who showed that, upon instruction by environmental signals, pluripotent stem cells exhibit developmental plasticity and transdifferentiate into alternative cell types. Two recent studies challenging this view have suggested that under selection pressure bone marrow cells or neural stem cells can fuse with embryonic stem cells (ES cells) in cultures and that bone marrow cells could fuse with ES cells, adopt an ES cell phenotype. In this in vitro study mixed bone marrow cells were used, and the frequency of fusion was 2 to 11 per 106 bone marrow cells. The Sca Lin_ fraction of bone marrow cells did not increase the frequency of fusion, suggesting that HSC were unlikely to be involved in the fusion event. Similarly, neural stem cells fused with ES cells and subsequently differentiated too many cell types. The frequency of fusion was in the range of 104 to 105 per brain cell [45].
PCNA expression is an index of renal regeneration. The administration of umbilical cord blood HSCs significantly increased the number of PCNA, positive cells, suggesting that UC-CD34+ strongly promoted tubular cell proliferation while inhibiting cell apoptosis that results resembled to [46].
On the other hand from the present study histopathological examination of renal tissue samples of ARF group showed increased congestion, increased cellularity of the glomeruli and fibrin deposition. There was also patchy tubular atrophy and necrosis. On the other hand normal medullary tubules with vacuolar degeneration as a late event were seen. Interstitial edema & mild inflammation also occurred. The present findings agreed with those of [47,48]; who demonstrate capillary loss that typically precedes the development of prominent fibrosis. After four week of umbilical cord CD34+ injection, there was improvement in the tissue of the kidney.
Moreover, the results of the present study showed increase in HGF, VEGF, IGF-1 growth factor gene and P53 that due to the mechanism of homing of hematopoietic stem cells in which HSPCs are recruited to the injured kidney and localize within injured capillaries and in the interstitium. Local production of cytokines including angiopoietins, vascular endothelial growth factors, hepatocyte growth factor and insulin like growth factors are generated promoting cellular repair by paracrine mechanisms which proved in our study. Also Pluripotent HSC possess the following complex characteristics: (i) differentiation potential for all hematopoietic lineages as demonstrated by clonal markers; (ii) high proliferative potential leading to as much as 100% donor-derived hematopoietic engraftment; (iii) long-term activity throughout the lifespan of the individual; and (iv) self-renewal as demonstrated by in vivo serial transplantations. And, although these stem cells have been phenotypically characterized for cell surface molecule expression, the gold standard in defining a HSC is only through in vivo Transplantation [49].
Further studies will be required to determine whether transplanted HSC can accelerate functional recovery after renal injury. Because HSC are more readily available compared with other somatic stem cells they can potentially be utilized as a source for cell replacement therapy.
In conclusion, we demonstrate here that systematically administered umbilical blood mobilized human HSCs reduce mortality and promote rapid renal repair and regeneration of the kidney by paracrine mechanisms directed at peritubular capillaries. These findings support human umbilical cord blood primitive hematopoietic cells as a promising therapeutic strategy for treatment of acute kidney diseases, and in the prevention of chronic kidney diseases.
Acknowledgements
Authors are thankful to the Unit of Biochemistry and Molecular Biology at the Medical Biochemistry Department, Faculty of Medicine, Cairo University, Egypt and clinical pathology department, faculty of medicine, Tanta University, Egypt for providing laboratory facilities and instruments to carry out this work.
Disclosure of conflict of interest
None.
References
- 1.Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med. 2004;351:159–169. doi: 10.1056/NEJMra032401. [DOI] [PubMed] [Google Scholar]
- 2.Gill N, Nally JV Jr, Fatica RA. Renal failure secondary to acute tubular necrosis: epidemiology, diagnosis, and management. Chest. 2005;128:2847–2863. doi: 10.1378/chest.128.4.2847. [DOI] [PubMed] [Google Scholar]
- 3.Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P, Hiesmayr M. Minimal changes of serum Creatinine predict prognosis in patients after cardiothoracic surgery: a Prospective cohort study. J Am Soc Nephrol. 2004;15:1597–1605. doi: 10.1097/01.asn.0000130340.93930.dd. [DOI] [PubMed] [Google Scholar]
- 4.Poulsom R, Forbes SJ, Hodivala-Dilke K, Ryan E, Wyles S, Navaratnarasah S, Jeffery R, Hunt T, Alison M, Cook T, Pusey C, Wright NA. Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol. 2001;195:229–235. doi: 10.1002/path.976. [DOI] [PubMed] [Google Scholar]
- 5.Burst VR, Gillis M, Pütsch F, Herzog R, Fischer JH, Heid P, Müller-Ehmsen J, Schenk K, Fries JW, Baldamus CA, Benzing T. Poor cell survival limits the beneficial Impact of mesenchymal stem cell transplantation on acute kidney injury. Nephron Exp Nephrol. 2010;114:e107–e116. doi: 10.1159/000262318. [DOI] [PubMed] [Google Scholar]
- 6.Togel F, Isaac J, Westenfelder C. Hematopoietic stem cell mobilization- associated granulocytosis severely worsens acute renal failure. J Am Soc Nephrol. 2004;15:1261–1267. doi: 10.1097/01.asn.0000123692.01237.0a. [DOI] [PubMed] [Google Scholar]
- 7.Bi B, Schmitt R, Israilova M, Nishio H, Cantley LG. Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol. 2007;18:2486–2496. doi: 10.1681/ASN.2007020140. [DOI] [PubMed] [Google Scholar]
- 8.Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86:897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- 9.Li B, Cohen A, Hudson TE, Motlagh D, Amrani DL, Duffield JS. Mobilized human hematopoietic Stem/progenitor cells promote kidney repair after ischemia/reperfusion injury. Circulation. 2010;121:2211–2220. doi: 10.1161/CIRCULATIONAHA.109.928796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 11.Uchida N, Weissman IL. Searching for hematopoietic stem cells: evidence that Thy-1.1lo Lin-Sca-1+cells are the only stem cells inC57BL/Ka-Thy-1.1 bone marrow. J Exp Med. 1992;175:175–184. doi: 10.1084/jem.175.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrison SJ, Wandycz AM, Hemmati HD, Wright DE, Weissman IL. Identification of a lineage of multipotent hematopoietic progenitors. Development. 1997;124:1929–1939. doi: 10.1242/dev.124.10.1929. [DOI] [PubMed] [Google Scholar]
- 13.Wolf NS, Kone A, Priestley GV, Bartelmez SH. In vivo and in vitro Characterization of long-term repopulating primitive hematopoietic cells Isolated by sequential Hoechst 33342- rhodamine 123 FACS selection. Exp Hematol. 1993;21:614–622. [PubMed] [Google Scholar]
- 14.Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M. Purified hematopoietic Stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 15.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infracted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 16.Okamoto R, Yajima T, Yamazaki M, Kanai T, Mukai M, Okamoto S, Ikeda Y, Hibi T, Inazawa J, Watanabe M. Damaged epithelia Regenerated by bone marrow-derived cells in the human gastrointestinal tract. Nat Med. 2002;8:1011–1017. doi: 10.1038/nm755. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu K, Sugiyama S, Aikawa M, Fukumoto Y, Rabkin E, Libby P, Mitchell RN. Host bone-marrow cells are a source of donor intimal smooth- muscle- like cells in murine aortic transplant arteriopathy. Nat Med. 2001;7:738–741. doi: 10.1038/89121. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 19.Anderson DJ, Gage FH, Weissman IL. Can stem cells cross lineage boundaries? Nat Med. 2001;7:393–395. doi: 10.1038/86439. [DOI] [PubMed] [Google Scholar]
- 20.Venkatachalam MA, Bernard DB, Donohoe JF, Levinsky NG. Ischemic damage and repair in the rat proximal tubule: differences among the S1, S2, and S3 segments. Kidney Int. 1978;14:31–49. doi: 10.1038/ki.1978.87. [DOI] [PubMed] [Google Scholar]
- 21.Jennische E, Andersson G. Selective damage to S3-segments in post-ischemic kidney as demonstrated by a simple histochemical method. Acta Pathol Microbiol Immunol Scand A. 1986;94:167–168. doi: 10.1111/j.1699-0463.1986.tb02981.x. [DOI] [PubMed] [Google Scholar]
- 22.Gobe G, Willgoss D, Hogg N, Schoch E, Endre Z. Cell survival or death in renal tubular epithelium after ischemia- reperfusion injury. Kidney Int. 1999;56:1299–1304. doi: 10.1046/j.1523-1755.1999.00701.x. [DOI] [PubMed] [Google Scholar]
- 23.Nigam S, Lieberthal W. Acute renal failure. III. The role of growth factors in the process of renal regeneration and repair. Am J Physiol Renal Physiol. 2000;279:F3–F11. doi: 10.1152/ajprenal.2000.279.1.F3. [DOI] [PubMed] [Google Scholar]
- 24.Safirstein R. Gene expression in nephrotoxic and ischemic acute renal failure. J Am Soc Nephrol. 1994;4:1387–1395. doi: 10.1681/ASN.V471387. [DOI] [PubMed] [Google Scholar]
- 25.Naghizadeh B, Boroushaki TM, Mashhadian VN, Mansouri MS. Protective Effects of Crocin against Cisplatin-Induced Acute Renal Failure and Oxidative Stress in Rats. Iran Biomed J. 2008;12:93–100. [PubMed] [Google Scholar]
- 26.Yadav CY, Srivastava ND, Saini V, Sighal S, Kumar S, Seth KA, Ghelani KT. Experimental Studies of Ficus religiosa (L) Latex for preventive and curative effect against cisplatin induced nephrotoxicity in wistar rats. J Chem Pharm Res. 2011;3:621–627. [Google Scholar]
- 27.Ravindra P, Bhiwgade AD, Kulkarni S, Rataboli VP, Dhume YC. Cisplatin induced histological changes in renal tissue of rat. Journal of Cell and Animal Biology. 2010;4:108–111. [Google Scholar]
- 28.Yadav S, Sahu P, Chaurasiya A. Role of cyamopsis tetra gonoloba against cisplatin induved genotoxicity: analysis of micronucleus and Chromosome aberration in vivo. J Bio Innov. 2013;2:184–193. [Google Scholar]
- 29.Arany I, Safirstein RL. Cisplatin nephrotoxicity. Semin Nephrol. 2003;23:460–464. doi: 10.1016/s0270-9295(03)00089-5. [DOI] [PubMed] [Google Scholar]
- 30.Kawai Y, Nakao T, Kunimura N, Kohda Y, Gemba M. Relationship of intracellular calcium and oxygen radicals to cisplatin-related renal cell injury. J Pharmacol Sci. 2006;100:65–72. doi: 10.1254/jphs.fp0050661. [DOI] [PubMed] [Google Scholar]
- 31.Buzzi FC, Fracasso M, Filho VC, Escarcena R, Del Olmo E, San Feliciano A. New antinociceptive agents related to dihydrosphingosine. Pharmacol Rep. 2010;62:849–857. doi: 10.1016/s1734-1140(10)70344-3. [DOI] [PubMed] [Google Scholar]
- 32.Muthuraman A, Sood S, Singla SK, Rana A, Singh A, Singh A, Singh J. Ameliorative effect of flunarizine in cisplatin-induced acute renal failure via mitochondrial permeability transition pore inactivation in rats. Naunyn Schmiedebergs Arch Pharmacol. 2011;383:57–64. doi: 10.1007/s00210-010-0572-z. [DOI] [PubMed] [Google Scholar]
- 33.Morigi M, Imberti B, Zoja C, Corna D, Tomasoni S, Abbate M, Rottoli D, Angioletti S, Benigni A, Perico N, Alison M, Remuzzi G. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004;15:1794–1804. doi: 10.1097/01.asn.0000128974.07460.34. [DOI] [PubMed] [Google Scholar]
- 34.Morigi M, Introna M, Imberti B, Corna D, Abbate M, Rota C, Rottoli D, Benigni A, Perico N, Zoja C, Rambaldi A, Remuzzi A, Remuzzi G. Human bone marrow mesenchymal Stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cells. 2008;26:2075–2082. doi: 10.1634/stemcells.2007-0795. [DOI] [PubMed] [Google Scholar]
- 35.Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: a review. Am J Med Sci. 2007;334:115–124. doi: 10.1097/MAJ.0b013e31812dfe1e. [DOI] [PubMed] [Google Scholar]
- 36.Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Mechanisms of Cisplatin Nephrotoxicity. Toxins (Basel) 2010;2:2490–2518. doi: 10.3390/toxins2112490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin F, Cordes K, Li L, Hood L, Couser WG, Shankland SJ, Igarashi P. Hematopoietic stem cells contribute to the regeneration of renal tubules after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol. 2003;14:1188–1199. doi: 10.1097/01.asn.0000061595.28546.a0. [DOI] [PubMed] [Google Scholar]
- 38.Fang TC, Otto WR, Rao J, Jeffery R, Hunt T, Alison MR, Cook HT, Wright NA, Poulsom R. Hematopoietic lineage-committed bone marrow cells, but not cloned cultured mesenchymal stem cells, contribute to regeneration of renal tubular epithelium after HgCl 2-induced acute tubular injury. Cell Prolif. 2008;41:575–591. doi: 10.1111/j.1365-2184.2008.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fang TC, Otto WR, Jeffery R, Hunt T, Alison MR, Cook HT, Wright NA, Poulsom R. Exogenous bone marrow cells do not rescue non-irradiated mice from acute renal tubular damage caused by HgCl2, despite establishment of chimeras and cell proliferation in bone marrow and spleen. Cell Prolif. 2008;41:592–606. doi: 10.1111/j.1365-2184.2008.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li L, Black R, Ma Z, Yang Q, Wang A, Lin F. Use of mouse hematopoietic stem and progenitor cells to treat acute kidney injury. Am J Physiol Renal Physiol. 2012;302:F9–F19. doi: 10.1152/ajprenal.00377.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson DJ, Gage FH, Weismann IL. Can stem cells cross lineage boundaries? Nat Med. 2001;7:393–395. doi: 10.1038/86439. [DOI] [PubMed] [Google Scholar]
- 42.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A. 2001;98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weismann IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 44.Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- 45.Ying QL, Nichols J, Evans EP, Smith AG. Changing potency by spontaneous fusion. Nature. 2002;416:545–548. doi: 10.1038/nature729. [DOI] [PubMed] [Google Scholar]
- 46.Li B, Cohen A, Hudson TE, Motlagh D, Amrani DL, Duffield JS. Mobilized Human Hematopoietic Stem/Progenitor Cells Promote Kidney Repair Following Ischemia Reperfusion Injury. Circulation. 2010;121:2211–2220. doi: 10.1161/CIRCULATIONAHA.109.928796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol. 2001;281:F887–899. doi: 10.1152/ajprenal.2001.281.5.F887. [DOI] [PubMed] [Google Scholar]
- 48.Yuan HT, Li XZ, Pitera JE, Long DA, Woolf AS. Peritubular capillary loss after mouse acute nephrotoxicity correlates with down-regulation of vascular endothelial growth factor-A and hypoxia-inducible factor-1 alpha. Am J Pathol. 2003;163:2289–2301. doi: 10.1016/s0002-9440(10)63586-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burst V, Putsch F, Kubacki T, Volker AL, Bartram PM, Muller UR, Gillis M, Kurschat EC, Grundmann F, Muller-Ehmsen J, Benzing T, Teschner S. Survival and distribution of injected hematopoietic stem cells in acute kidney injury. Nephrol Dial Transplant. 2012;28:s1–8. doi: 10.1093/ndt/gfs513. [DOI] [PubMed] [Google Scholar]


