Renin contributes to HIV replication in T cells via (P) RRPLZF signaling cascade and cleavage of Gag polyproteins.
Keywords: Vitamin D receptors, Aspartyl protease
Abstract
HIV is known to subvert cellular machinery to enhance its replication. Recently, HIV has been reported to enhance TC renin expression. We hypothesized that HIV induces and maintains high renin expression to promote its own replication in TCs. Renin enhanced HIV replication in TCs in a dose-dependent manner. (P)RR-deficient TCs, as well as those lacking renin, displayed attenuated NF-κB activity and HIV replication. TCs treated with renin and Hpr displayed activation of the (P)RR-PLZF protein signaling cascade. Renin, HIV, and Hpr activated the PI3K pathway. Both renin and Hpr cleaved Agt (a renin substrate) to Ang I and also cleaved Gag polyproteins (protease substrate) to p24. Furthermore, aliskiren, a renin inhibitor, reduced renin- and Hpr-induced cleavage of Agt and Gag polyproteins. These findings indicate that renin contributes to HIV replication in TCs via the (P)RR-PLZF signaling cascade and through cleavage of the Gag polyproteins.
Introduction
Renin, the prime initiation molecule of the RAS was identified more than a century ago [1]; however, its receptor (P)RR was identified only 2 decades ago [1, 2]. Before the identification of (P)RR, renin was known for its hemodynamic contributions through the generation of Ang II [3]. Currently, renin is being investigated for its direct cellular effects in cardiovascular biology [4]. Several investigators have revealed that renin directly contributes to the end-organ damage caused by hypertension and diabetes [5–7].
(P)RR is an integral component of mammalian vacuolar type H+-ATPase (V-ATPase) [2] and participates in each step of membrane trafficking from entry of molecules by receptor-mediated endocytosis to their processing via lysosomal/autophagosomal compartments. Moreover, (P)RR works as an adaptor protein between the Wnt receptor complex and V-ATPase [8]. Renin binding to (P)RR induces nuclear translocation of the PLZF protein, repressing (P)RR and simultaneous activation of PI-3K [9, 10].
(P)RR has been demonstrated to induce smooth-muscle cell proliferation and hypertrophy through the Src, ERK, and Akt pathways [11]. Conversely, blocking (P)RR from binding to its ligands prevents the development of cardiac fibrosis and diabetic nephropathy [12–15]. (P)RR has also been shown to enhance production of TNF-α and IL-1β, independent of its Ang II generation [13–15].
TCs have been reported to display endogenous RAS, and its activation contributes to the development of hypertension and tubulointerstitial fibrosis [16, 17]. Recently, we reported that HIV enhances renin generation in several cell types, including CD4 TCs [18–20]. HIV-infected TCs were found to have down-regulated protein levels of VDR and treatment of HIV-infected TCs with a VDA not only up-regulated VDR expression, but also down-regulated renin expression [18]. In several disease models, VDAs have been reported to down-regulate cellular renin expression [21, 22].
In the current study, we found that renin enhances HIV replication through its interaction with (P)RR, as well as by mimicking Hpr. Renin-induced (P)RR activation was associated with activation of the PLZF-PI-3K pathway, leading to enhanced NF-κB activity. Interestingly, Hpr also interacted with (P)RR and enhanced downstream signaling, including the activation of HIV-LTR. Hpr cleaved a renin-specific substrate, Agt, while renin cleaved Gag polyproteins. These findings indicate that renin enhances HIV replication through (P)RR and cleavage of Gag polyproteins.
MATERIALS AND METHODS
Human TCs
TCs were isolated from blood obtained from healthy volunteers (New York Blood Bank, New York, NY, USA). In brief, PBMCs were harvested by the standard technique [23]: the TCs were isolated from the PBMCs by passing them through a TC-negative selection column (Invitrogen, Oslo, Norway) and were primed before their use (IL-2, 100 U/mL and PHA-P, 5.0 μg/mL).
MTT assay
To determine the effect of renin on TC viability, we treated the TCs with various concentrations of renin (0, 0.1, 1.0, 10.0, 50, and 100 nM) for 24 h. Subsequently, the cells were examined for viability by MTT assay [23]. There was no difference in the viability of the control cells and TCs treated with up to 5 nM renin.
HIV infection of TCs
Activated TCs were pulsed with a primary strain of HIV-1 (HIV-192HT599, SI isolates, 0.5 IU [MOI]; NIH AIDS Reagent Program, Bethesda, MD, USA) for 2 h, followed by trypsinization and extensive washing to remove adherent HIV particles, and then the TCs were reincubated in medium. HIV-1 replication was measured by harvesting control (including negative) cells, experimental cells, and incubation medium. Protein and RNA were extracted from the cells. cDNAs were probed for LTR and Gag. Protein blots were probed for Gag (NIH AIDS Reagent Program) and reprobed for actin. The p24 antigen concentrations in the incubation media were measured by p24 ELISA, according to the manufacturer's instructions.
IP
The protein lysates were immunoprecipitated after addition of 10 μL of polyclonal Ab to PLZF (Santa Cruz Biotechnology, Dallas, TX, USA). The immune complexes were then harvested by using 25 μL of protein A+G Sepharose beads (GE Healthcare Life Sciences, Pittsburgh, PA, USA) in RIPA buffer. The IP was performed at 4°C, for 4 h, on a rotating platform, and the protein (A+G)-precipitated proteins were pelleted down by centrifugation at 4500 rpm for 10 min at 4°C. Next, the protein pellet was washed 3 times with 1 mL of cold RIPA lysis buffer, followed by centrifugation each time for 10 min at 2500 rpm in a microfuge. After they were washed, the beads were resuspended in 30 μL of lysis buffer to which SDS-PAGE sample buffer (30 μL) was added, and the samples were boiled at 100°C, followed by SDS-PAGE on Criterion Gel 4–20% Tris-HCl polyacrylamide gels (Bio-Rad, Hercules, CA, USA) and analysis by Western immunoblot with the specified antibodies.
Preparation of nuclear extracts and EMSA
Nuclear extracts from control and experimental cells (1×107) were prepared. The NF-κB DNA-binding protein detection system kit (Affymetrix, Santa Clara, CA, USA) was used for the EMSA, with aliquots of 1 μg extract. Briefly, the protein-binding biotinylated DNA probe (NF-κB) was incubated with the nuclear extracts prepared from the control and experimental cells, according to the manufacturer's protocol (Panomics, Redwood City, CA, USA). The DNA–protein binding reactions were performed at room temperature for 10 min in 10 mM Tris-HCl (pH 7.9), 50 mM NaCl, 5 mM MgCl2, 1 mM EDTA, and 1 mM DTT, plus 1 μg poly (dI-dC), 5% (v:v) glycerol, and ∼10 ng biotinylated NF-κB probe. Protein DNA complexes were resolved from protein-free DNA on 6% polyacrylamide gels (Invitrogen) at 4°C in 50 mM Tris (pH 8.30) and 2 mM EDTA. The DNA–protein complexes and the remaining gel contents were transferred to Biodyne B membranes (Pall Corp., Ann Arbor, MI, USA) for 60 min at 300 mA. The membranes now containing the DNA–protein complexes were UV cross-linked, and chemiluminescence detection of biotinylated DNA was performed with an EMSA kit (Panomics/Affymetrix).
Preparation of Hpr and pr55gag-polyprotein lysates
Plasmid pr55gag was prepared from transformed JM109 cells, as described in the protocol [24], and was obtained from NIH AIDS Reagent pDAB72. Hpr pCDNA3-PR was obtained from Addgene (Cambridge, MA, USA) and incubated in ampicillin-resistant Luria-Bertani medium for 10 h at 37°C with rotation. The plasmid was prepared with a spin miniprep kit (QIAprep; Qiagen, Valencia CA, USA), and concentration was calculated. Six micrograms of the plasmid was transfected into TCs for 24 h. Cell extracts were prepared by suspending the pelleted cells in 5 mL 20 mM KH2PO4 (pH 7.2) and 150 mM NaCl (PBS buffer). Cell debris was removed by centrifuging at 15,000 g. The resulting lysate was stored in aliquots at −70°C.
Western blot analyses
TCs were lysed in RIPA buffer containing 50 mM Tris-Cl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1% NP-40, 0.25% deoxycholate, 0.1% SDS, 1× protease inhibitor cocktail I (Calbiochem, EMD Biosciences, Gibbstan, NJ, USA), 1 mM PMSF, and 0.2 mM sodium orthovanadate. Protein concentrations were determined with the Bio-Rad Protein Assay kit (Bio-Rad). Protein lysates (20 μg) were separated on a 15% polyacrylamide gel (Bio-Rad) and transferred onto a nitrocellulose membrane with a miniblot apparatus (Bio-Rad). Nitrocellulose membranes were then subjected to immunostaining with primary antibodies against VDR (mouse monoclonal; Santa Cruz Biotechnology), renin (monoclonal; Santa Cruz Biotechnology), (P)RR (ATP6IP2; Abcam, Cambridge, MA, USA), Hpr (mouse monoclonal; Santa Cruz Biotechnology), and Gag (NIH AIDS Reagent Program) and subsequently with the appropriate HRP-labeled secondary antibodies. The blots were developed with a chemiluminescence detection kit (Pierce Biotechnology, Rockford, IL, USA) and exposed to X-ray film (Eastman Kodak Co., Rochester, NY, USA). Equal protein loading was confirmed by stripping the blot and reprobing it for actin protein with a β-actin antibody (Santa Cruz Biotechnology) on the same Western blots.
RT-PCR Analysis
Control and experimental TCs were used to quantify LTR and Gag mRNA expression. RNA was extracted with TRIzol (Invitrogen/Life Technologies, Grand Island, NY, USA). For cDNA synthesis, 2 μg of the total RNA was preincubated with 2 ng of random hexamer (Invitrogen) at 65°C for 5 min. Subsequently, 8 μL of the RT reaction mixture containing cloned avian myeloblastosis virus RT, 0.5 mM each of the mixed nucleotides, 0.01 M DTT, and 1000 U/mL RNAsin (Invitrogen) were incubated at 42°C for 50 min. For the negative control, a reaction mixture without RNA or RT was used. Samples were subsequently incubated at 85°C for 5 min to inactivate the RT.
Quantitative PCR was performed in an ABI Prism 7900HT sequence detection system (Life Technologies) with the following primer sequences: LTR forward, GCTAACTAGGGAACCCACTG, reverse, GCTAGAGATTTTCCACACTGA and GAG forward, AATCCACCTATCCCAGTAGGAG, reverse, TGGTCCTTGTCTTATATCCAGAAT.
SYBR green was used as the detector and ROX (Life Technologies) as a stabilizing dye. The results (mean ± sd) represent at least 3 sets of experiments, as described in the legend. The data were analyzed by using the CT (ΔΔCT) method (Figs. 1–3). Differences in CT were used to quantify the relative amount of PCR target contained within each well. The data are expressed as relative mRNA expression in reference to the control, normalized to the quantity of RNA input by performing measurements on the endogenous reference gene GAPDH.
Figure 1. Renin modulates HIV replication.
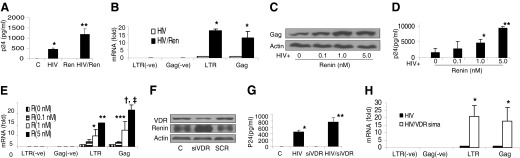
(A) Control TCs (C) and HIV-pulsed TCs (HIV) were incubated in medium containing either buffer or renin (0.1 nM) for 24 h (n=4). Aliquots of the incubation media were collected for p24 assay by ELISA. *P < 0.1, compared to the control; **P < 0.01, compared to HIV alone. (B) RNA was extracted from the cells treated in (A) and probed for cellular mRNA expression for LTR and Gag by positive and negative probes. *P < 0.001, compared to respective HIV alone. (C) HIV-pulsed TCs were incubated in the presence of various concentrations of renin (0, 0.1, 1.0, and 5.0 nM) for 24 h (n=4). At the end of the incubation period, protein blots were probed for Gag and were reprobed for actins. Representative gels are displayed. (D) Samples of media were collected from the cells in (C) and assayed for p24 by ELISA (n=4). Cumulative data are shown in the bar graph. *P < 0.05, compared to control; **P < 0.01, compared to other variables. (E) Cellular RNA was extracted from the cells in (C) and probed for mRNA expression for LTR and Gag by positive and negative probes. Cumulative data (n=4) are displayed in the bar graph. *P < 0.05, compared to 0 renin (R); **P < 0.01, compared to all other variables; ***P < 0.05, compared to R doses 0 and 0.1 nM; †P < 0.001 compared to R doses 0 and 0.1 nM; and ‡P < 0.05 compared to R dose 1 nM). (F) TCs were transfected with siVDR or SCR. Protein blots of control, siVDR, and SCR TCs were probed for VDR. The same blots were probed for renin and actin. Representative gels of control and experimental cells are shown. (G) Control and siVDR TCs were pulsed with HIV or treated with PBS buffer and then incubated in RPMI medium for 24 h (n=3). Subsequently, incubation media were collected for p24 ELISA. Cumulative data are shown in the bar graph. *P < 0.01 compared to control; **P < 0.05 compared to HIV alone. (H) Cellular RNA was extracted from the cells in (G) and probed for LTR and Gag. Cumulative data are shown in the bar graph. P < 0.01 compared to respective HIV alone.
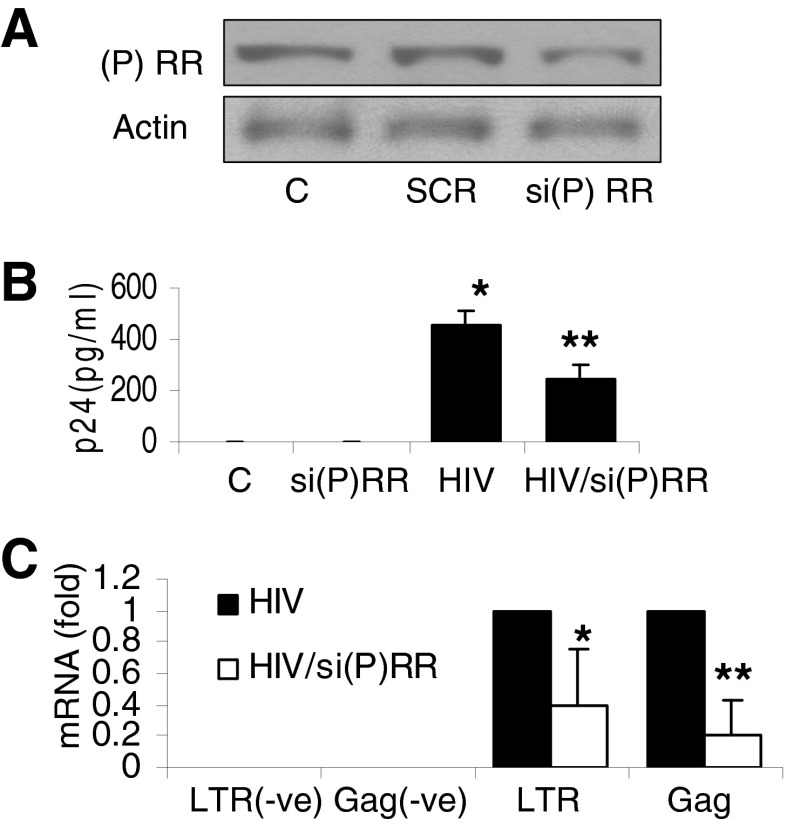
Figure 3. Role of (P)RR in HIV replication.
(A) TCs were transfected with either si(P)RR or SCR. Protein blots were probed for (P)RR, and the same blots were reprobed for actin. Representative gels of control and transfected cells are shown. (B) TCs and si(P)RR TCs were either pulsed with HIV or treated with buffer and then incubated in medium for 24 h (n=3). Aliquots of media were collected for a p24 assay by ELISA. Results (mean ± sd) are from 3 sets of experiments. *P < 0.001, compared to control; **P < 0.01, compared to HIV alone. (C) Cellular RNA from the cells in (B) was probed for mRNA expression of Gag and LTR. Cumulative data (n=3) are shown in the bar graph. *P < 0.05, compared to respective HIV alone; **P < 0.01, compared to respective HIV alone.
Ang I ELISA
Aspartyl protease (renin and Hpr) activity was measured as Ang1 formed in the presence of recombinant human renin (cat. no. 10006217; Cayman Chemical, Ann Arbor, MI, USA) and Hpr. Briefly, Agt (GWB-FE60B8; GenWay Biotech, Inc., San Diego, CA, USA) was used as a substrate for the renin. Samples were incubated with and without Agt (1 μM) at 37°C for 1 h followed by measurement of Ang I with an ELISA kit (cat. no. ADI 900-203; Enzo Life Sciences, Farmingdale, NY, USA).
Gag proteolytic activity
A protease assay was performed as described elsewhere [24]. Gag translation extract (100 μL) was mixed with either 600 μL of Hpr-transduced cell lysate or recombinant human renin (1 nM; Cayman Chemical) in 100 μL PBS and incubated for 60 min at 37°C. Subsequently, aliquots were assayed for p24 by ELISA (Lenti-X p24 Rapid Titer Kit, Cat. No 632200; Clontech, Mountain View, CA, USA).
Immunofluorescence detection of nuclear p-p65
Control and experimental TCs were fixed and permeabilized with a buffer containing 0.02% Triton X-100 and 4% formaldehyde in PBS. Fixed cells were washed 3 times in PBS and blocked in 1% BSA for 30 min at 37°C. The cells were colabeled with Hoechst (nuclear) and anti NF-κB p-p65 rabbit antibody (Santa Cruz Biotechnology) and Alexa-conjugated goat anti-rabbit secondary antibody (Invitrogen). Double labeling was indicated by purple staining. Specific staining was visualized with an inverted 1X 70 fluorescence microscope equipped with a Cook Sensicom ER camera (Olympus America, Melville, NY, USA). Final images were processed with PhotoShop (Adobe, San Jose, CA, USA).
Silencing of VDR, Renin, and (P)RR
TCs were transfected with 20 nM VDR, renin, (P)RR, or control siRNA (Santa Cruz Biotechnology) via Siport Neofax transfection reagent (Life Technologies) and incubated in reduced-serum medium (optiMEM, Life Technologies) for 48 h. Control and transfected cells were used in control and experimental conditions.
Statistical analysis
For comparison of the means of 2 groups, the unpaired t test was used. For values between multiple groups, ANOVA was used to calculate the probability (P). Statistical significance was defined as P < 0.05. Results are presented as the mean ± sd.
RESULTS
Renin modulates HIV replication
To determine the effect of renin, control and HIV-pulsed TCs were incubated in medium containing either buffer or renin (1 nM) for 24 h. Subsequently, samples of the media were collected for p24 ELISA, and RNA was extracted from the cells and amplified with primers for LTR and Gag by real-time PCR. As shown in Fig. 1A, renin enhanced p24 content in HIV-pulsed cells by 3-fold; similarly, renin enhanced LTR and Gag expressions in HIV-pulsed cells by 15- and 10-fold, respectively (Fig. 1B).
To determine the dose–response effect of renin, HIV-pulsed TCs were incubated in medium containing various concentrations of renin (0, 0.1, 1.0, and 5.0 nM) for 24 h (n=4). Subsequently, incubation media were collected for p24 ELISA, and the cells were harvested for protein and RNA extraction. Protein blots were probed for Gag and reprobed for actin. cDNA was probed for LTR and Gag. Representative gels displaying expressions of Gag and actin in control and experimental cells are shown in Fig. 1C. Cumulative data on the dose–response effect of renin on the p24 content of HIV-pulsed TCs are shown in Fig. 1D. Renin enhanced p24 levels in HIV-pulsed TCs in a dose-dependent manner. Similarly, it enhanced the expression of both LTR and Gag in HIV-pulsed TCs (Fig. 1E). We evaluated the effect of Ang II (10−8 to 10−6 M) on HIV replication, and found that it had none (data not shown).
To determine the effect of an enhanced endogenous renin state (through silencing VDR) on TC HIV replication, TCs were transfected with either siRNA-VDR (siVDR) or scrambled siRNA (SCR). Protein blots of control, siVDR, and SCR TCs were probed for VDR and reprobed for renin and actin. Representative gels from control and experimental cells are shown in Fig. 1F. TCs silenced for VDR displayed enhanced expression of renin.
In parallel sets of experiments, control and siVDR TCs were pulsed with HIV or treated with buffer and then incubated in medium for 24 h (n=3). Subsequently, the incubation media were collected for p24 ELISA, the cells were harvested for RNA extraction, and PCR was performed for LTR and Gag expression. The HIV/siVDR TCs displayed higher (P<0.05) p24 levels than the HIV TCs (Fig. 1G). The HIV TCs silenced for VDR displayed higher expression of both LTR and Gag than the HIV TCs (Fig. 1H).
Lack of renin and inhibition of its proteolytic activity are associated with attenuated TC HIV replication
To determine the effect of the absence of endogenous renin on HIV replication in TCs, TCs were transfected with either siRen or SCR. Protein blots of control, siRen, and SCR TCs were probed for renin and reprobed for actin. Representative blots of control and experimental cells are shown in Fig. 2A. The control and siren TCs were pulsed with either HIV or buffer and then incubated in medium for 24 h; subsequently, the incubation media were assayed for p24 contents, and cDNA was used for PCR amplification. The HIV/siRen TCs displayed lower (P<0.05) p24 levels than the HIV TCs (Fig. 2B); similarly, the HIV/siRen TCs displayed more attenuated (P<0.01) expression of LTR and Gag than the HIV TCs (Fig. 2C).
Figure 2. Lack of renin or inhibition of its proteolytic activity are associated with attenuated TC HIV replication.
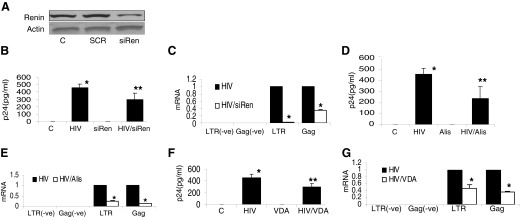
(A) TCs were transfected with either siRen or SCR. Protein blots of control, siRen, and SCR TCs were probed for renin, and the same blots were reprobed for actin. Representative gels of control and experimental cells are shown. (B) Control and siRen TCs were either pulsed with HIV or treated with buffer and then incubated in medium for 24 h (n=3). Samples of incubation media were assayed for p24 by ELISA. Cumulative data of 3 sets of experiments are shown in the bar graph. *P < 0.001 compared to control; **P < 0.05 compared to HIV alone. (C) Cellular RNA was extracted from the cells in (B) and probed for LTR and Gag. Cumulative data (n=3) are shown in the bar graph.*P < 0.01 compared to respective HIV alone. (D) Control and HIV-pulsed TCs were treated with buffer or aliskiren (1 μM) for 24 h (n=4). Aliquots of media were collected for p24 ELISA. Results (mean ± sd) represent 4 sets of experiments. *P < 0.001 compared to control; **P < 0.05, compared to HIV alone. (E) RNA was extracted from the cells harvested from (D) and probed for LTR and Gag. Cumulative data are shown in the bar graph. *P < 0.01, compared to HIV alone. (F) Control and HIV-pulsed TCs were incubated in medium containing either buffer or VDA (50 pM; EB1089) for 24 h (n=4). Aliquots of incubation media were collected for p24 assay by ELISA. Results (mean ± sd) represent 4 sets of experiments. *P < 0.001, compared to control; **P < 0.05, compared to HIV alone. (G) Cellular RNA was extracted from the cells in (F) and probed for LTR and Gag. Cumulative data (n=3) are shown in the bar graph.*P < 0.01, compared to respective HIV alone.
To determine the effect of inhibition of the proteolytic activity of renin, control and HIV-pulsed TCs were treated with either buffer or 1 μM aliskiren (a renin activity inhibitor) for 24 h (n=4) followed by collection of the incubation media for p24 ELISA and harvesting of cells for mRNA expression (LTR and Gag). Aliskiren partially attenuated (P<0.05) p24 levels in the HIV TCs (Fig. 2D); similarly, aliskiren inhibited (P<0.01) TC expression of LTR and Gag in the HIV TCs (Fig. 2E).
Because 1,25(OH)2D is a negative regulator of renin transcription, VDAs have been used to attenuate renin expression in a variety of cells [25]. To determine the effect of a VDA, control TCs and HIV-pulsed TCs were incubated in medium containing either buffer or a VDA (50 pM; EB1089) for 24 h (n=4), followed by collection of the incubation media for p24 ELISA and harvest of the cells for RNA extraction. VDA partially attenuated (P<0.01) p24 levels in HIV TCs (Fig. 2F). It also attenuated (P<0.01) LTR and Gag expression in HIV TCs (Fig. 2G).
Role of (P)RR in HIV replication
To determine the role of (P)RR in HIV replication, TCs were transfected with either si-(P)RR or SCR. Protein blots were probed for (P)RR and then reprobed for actin. Representative gels of control and experimental cells are shown in Fig. 3A. TCs and si(P)RR TCs were either pulsed with HIV or treated with buffer and then incubated in medium for 24 h (n=3) followed by assessment of HIV replication (p24 levels in the media and cellular mRNA expression of Gag and LTR). The HIV TCs silenced for (P)RR displayed lower (P<0.01) p24 levels when compared with levels in the HIV TCs (Fig. 3B). The HIV TCs lacking (P)RR displayed further attenuated expression of LTR (P<0.05) and Gag (P<0.01), compared with levels in the HIV TCs (Fig. 3C). These findings indicate that (P)RR plays a role in TC HIV replication.
Both renin and Hpr contribute to HIV replication through their proteolytic activities
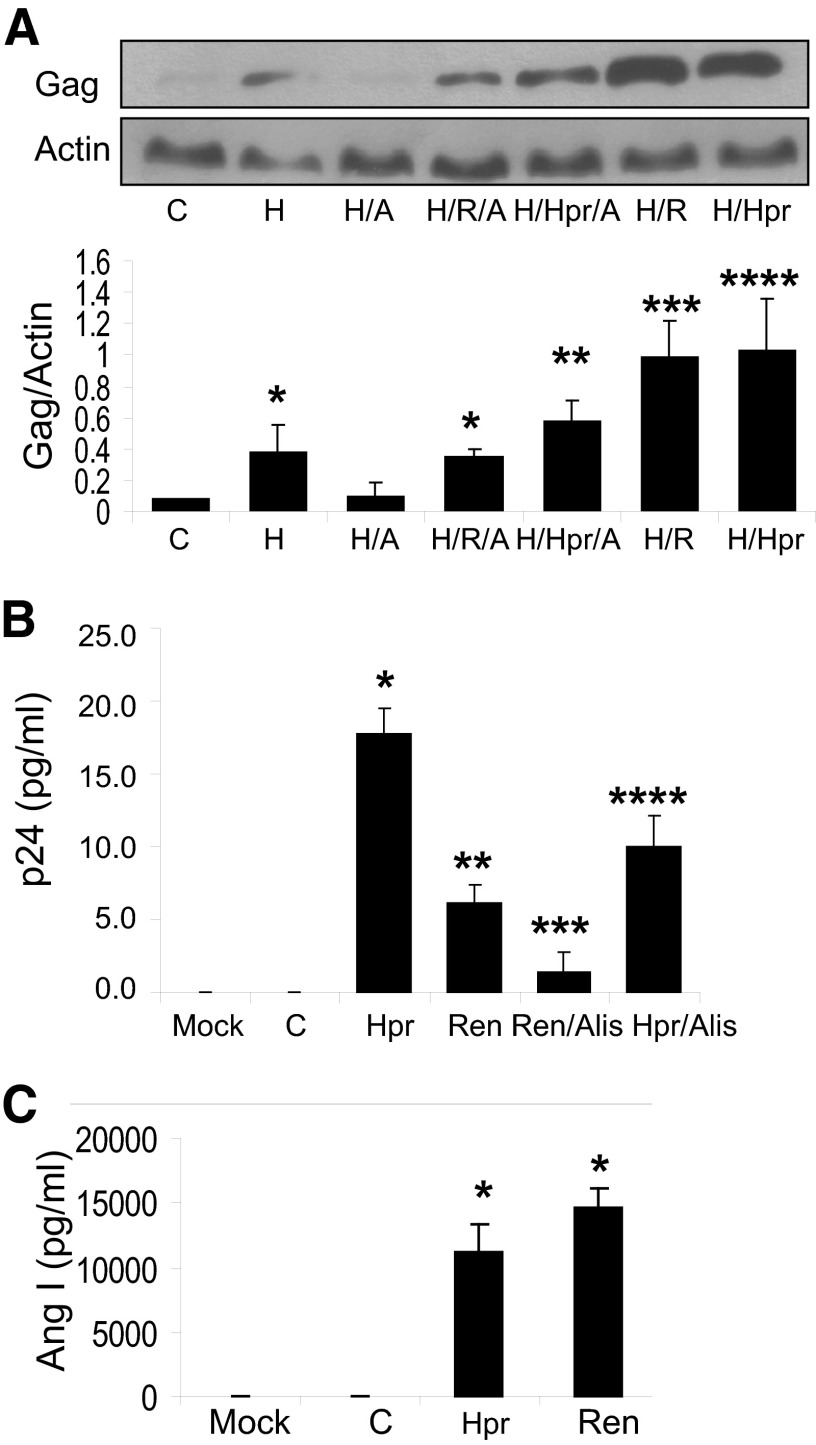
To determine the effect of overexpression of Hpr, control TCs and TCs transfected with the Hpr plasmid were pulsed with either HIV or buffer and then incubated in medium containing buffer or renin (R, 1 nM), aliskiren (A, 1 μM), or renin+aliskiren for 24 h (n=3). Protein blots were probed for Gag; the same blots were reprobed for actin. Representative gels are displayed in Fig. 4A. Cumulative densitometric data are shown in the bar graph. Both Hpr and renin enhanced (P<0.01) HIV replication. Aliskiren inhibited HIV replication in the basal (P<0.05) and Hpr/renin-stimulated states (P<0.01). These findings confirm that both Hpr and renin contribute to HIV replication through their proteolytic activities.
Figure 4. Role of renin and Hpr in enhancing HIV replication through their proteolytic activities.
(A) Control TCs and TCs overexpressing Hpr were either pulsed with HIV (H) or treated with buffer and then incubated in media containing either buffer or renin (R, 1 nM), aliskiren (A, 1 μM), or renin+aliskiren for 24 h (n=3). Protein blots were probed for Gag protein; the same blots were reprobed for actin. Representative gels are displayed. Cumulative densitometric data of Gag protein expression under control and experimental conditions are shown in the bar graph (n=3). *P < 0.05, compared to C and H/A; **P < 0.01, compared to H/Hpr; ***P < 0.01 compared H alone; and ****P < 0.01, compared to H alone. (B) Lysates of pr55 gag polyprotein-expressing TCs (100 μL) were incubated in medium containing buffer (C) or lysates of TCs overexpressing Hpr (600 μL), renin (1 nM), renin+aliskiren (Alis, 1 μM), or Hpr+Alis for 60 min (n=3). Aliquots of media were collected for p24 ELISA. Results (mean ± sd) are from 3 sets of experiments. *P < 0.001, compared to C; **P < 0.01, compared to C; ***P < 0.01, compared to Ren; and ****P < 0.01, compared to Hpr. (C) Aliquots of Agt were incubated in medium containing lysates of Hpr-expressing TCs (600 μL) and renin (1 nM) for 30 min (n=3). Subsequently, aliquots of media were collected for Ang I ELISA. Cumulative data are shown in the bar graph. *P < 0.001, compared to C.
To determine the effect of renin on cleavage of Gag polyproteins, lysates of pr55 gag polyprotein–expressing TCs were incubated for 60 min in medium containing buffer (C), lysates of cells overexpressing protease (Hpr), renin, renin+aliskiren, or Hpr+aliskiren (n=3). Aliquots of the media were collected for p24 ELISA. Hpr enhanced (P<0.001) cleavage of Gag polyproteins when compared to the control (Fig. 4B). Aliskiren inhibited (P<0.01) cleavage of Gag polyproteins in both the Hpr- and renin-treated states. Renin displayed only moderate (P<0.01) cleavage of Gag polyproteins. However, this difference in proteolytic activity between Hpr and renin may be related to the use of doses of renin and Hpr of various potencies.
To determine whether Hpr also has the potency to cleave renin substrate, aliquots of Agt were incubated in medium containing lysates of Hpr-expressing cells (600 μL) or renin (1 nM) for 30 min (n=3). Subsequently, samples of media were collected and assayed for Ang I ELISA. Both Hpr and renin showed cleavage of Agt (Fig. 4C). These findings indicate that Hpr has proteolytic activity similar to renin.
Role of PLZF pathway
We hypothesized that binding of renin to (P)RR causes dissociation of PLZF and activation of the PI3K pathway, as shown in the schematic display (Fig. 5A, post-renin). To validate our hypothesis, TCs were incubated in medium containing either renin (1 nM) or Hpr (600 μL) for the indicated times. Subsequently, IP of cell lysates with PLZF antibody was performed. IP fractions were probed for (P)RR, and PLZF (IgG as a contaminant served as the internal control for equal loading of samples). Representative gels are shown in Fig. 5B. Renin- and Hpr-treated cells showed dissociation of (P)RR from PLZF in a time-dependent manner.
Figure 5. Role of the PLZF pathway.
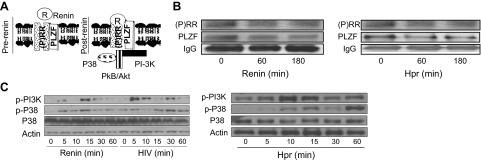
(A) Binding of renin to (P)RR, inducing dissociation of PLZF and activation of the PI3K pathway in the postrenin phase. (B) TCs were incubated in medium containing renin (1 nM) or Hpr (600 μL) for the indicated times. Subsequently, IP of cell lysates with the PLZF antibody was performed. The IP fractions were probed for (P)RR and PLZF. Representative gels are shown. (C) TCs were incubated in medium containing renin (1 nM) or Hpr (600 μL) or were pulsed with HIV for the indicated times. Protein blots were probed with p-PI3K and p-p38. The same blots were reprobed for p38 and actin. Representative gels are shown.
Since the PLZF pathway is known to activate PI3K and P38 signaling, TCs were either incubated in medium containing renin (1 nM) or Hpr (600 μL) or were pulsed with HIV for 2 h and then incubated in medium for various intervals up to 60 min for Western blot analysis of p-PI3K and p-p38. These blots were then reprobed for p38 and actin. Representative gels are shown in Fig. 5C. Renin, HIV, and Hpr, all activated the PI3K pathway.
Both lack of renin and (P)RR-deficient states inhibit the activation of NF-κB in TCs
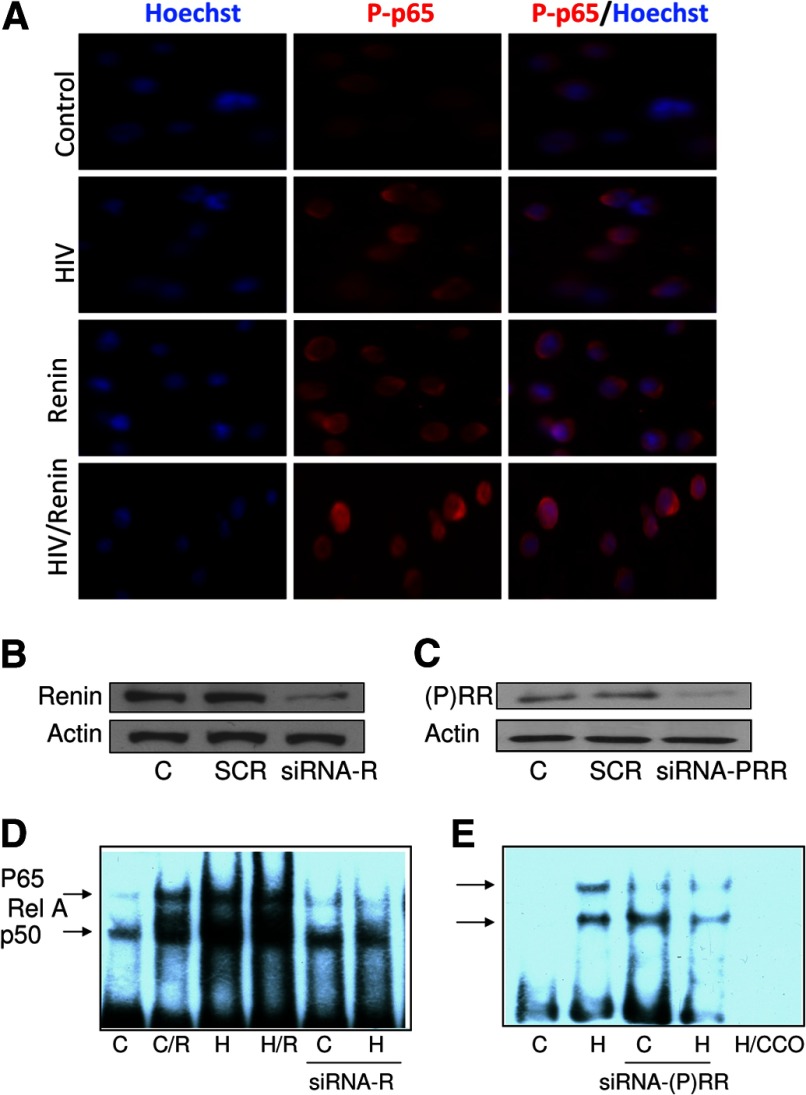
HIV has been reported to enhance its replication through the activity of NF-κBp65 in TCs [26]. To determine the effect of renin on TC NF-κBp65 activity (in basal and HIV milieus), TCs were either pulsed with HIV or buffer and then incubated in medium containing renin (1 nM) for 48 h. Subsequently, the cells were colabeled with Hoechst (nuclear stain) and p-p65 and examined under a fluorescence microscope. The control cells displayed minimal nuclear presence of p-p65, whereas both the renin- and HIV-treated TCs displayed a moderate nuclear presence of p-p65, indicating nuclear translocation of NF-κB. On the other hand, the TCs receiving combined treatment (renin and HIV) displayed a greater nuclear presence of p-p65 (Fig. 6A). These findings indicate that renin enhances HIV-induced translocation of NF-κB in TCs.
Figure 6. Both lack of renin and (P)RR deficiency inhibit the activation NF-κB in TCs.
(A) TCs were either pulsed with HIV or treated with buffer and then incubated in medium containing renin (1 nM) for 24 h. Subsequently, the cells were colabeled with Hoechst (nuclear stain) and phospho-p65 and examined under a fluorescence microscope. Representative microfluorographs are shown. Control cells displayed minimal nuclear presence of p-p65, whereas both renin- and HIV-treated TCs displayed a moderate nuclear presence of p-p65, thus indicating nuclear translocation of NF-κB. On the other hand, TCs receiving combined treatment (renin and HIV) displayed a maximum nuclear presence of p-p65. (B, C) TCs (C) were transfected with siRen, si(P)RR, or SCR. Protein blots of control, siRen, si(P)RR, or SCR TCs were probed with renin and (P)RR. The same blots were reprobed for actin. Representative gels are shown. (B) TCs showing partial silencing of renin. (C) TCs display silencing of (P)RR. (D) Control TCs and SiRen (R)-transfected TCs were pulsed with HIV (H) and then incubated in medium containing either buffer or renin (R, 1 nM) for 4 h (n=3). EMSA was performed on nuclear extracts from control and experimental cells. A representative gel is shown. Control (C), with renin (C/R), HIV-pulsed (H), HIV+renin (H/R), and cells silenced for renin (siRNA-R), including control (C) and HIV-pulsed cells (H). (E) Control TCs and siRNA-(P)RR transfected TCs were pulsed with HIV (H) and then incubated in medium for 4 h (n=3). EMSA was performed on nuclear extracts from control and experimental cells. A representative gel is shown.
To determine the role of renin and (P)RR in the activation of TC NF-κB, control TCs were transfected with siRen/si(P)RR or SCR. Protein blots of control cells, siRen, si(P)RR, and SCR were probed with renin and (P)RR. The same blots were reprobed for actin. Representative gels are shown in Fig. 6B and C.
To confirm the role of renin in NF-κB activity, control cells and siRNA-transfected TCs were pulsed with HIV and then incubated in medium containing either buffer or renin (1 nM) for 4 h (n=3). To evaluate the role of (P)RR in NF-κB activation, control TCs and si(P)RR-transfected TCs were pulsed with HIV and then incubated in medium for 4 h (n=3).
Nuclear extracts of both control and experimental cells were assayed for NF-κB by EMSA. Representative gels are shown in Fig. 6D and E. Both renin and HIV enhanced NF-κB activity; however, cells silenced for renin displayed attenuated NF-κB activity, both in the control and the HIV-stimulated states (Fig. 6D). Furthermore, TCs silenced for (P)RR also displayed attenuated NF-κB binding activity, both in control and HIV-pulsed conditions (Fig. 6E). These findings indicate that both renin and (P)RR contribute to HIV-induced TC NF-κB activation.
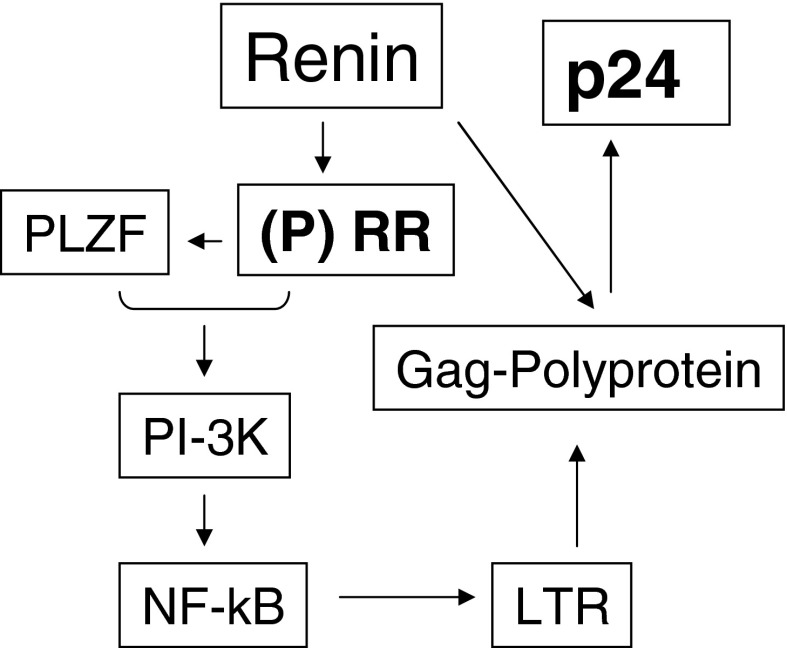
Figure 7 shows a representation of the proposed contribution of renin to HIV replication via (P)RR and Gag polyproteins.
Figure 7. Schematic representation of the proposed role of renin in HIV replication.
Interaction of renin with (P)RR leads to binding of (P)RR to PLZF and its translocation to the nucleus. The PLZF-activated PI3K pathway enhances NF-κB activity, resulting in its binding to the LTR promoter site and thus advancing the propagation of the Gag polyprotein. Both renin and Hpr cleave the Gag polyproteins which results in the generation of proviral proteins, including p24.
DISCUSSION
Both renin and Hpr are aspartyl proteases and are structurally similar [27]. Therefore, protease inhibitors in general, have been designed based on renin structure [27]. In addition, serum Ang I levels have been reported to display the bioavailability of protease inhibitors [28, 29]. Our findings are consistent with those reports. It has been speculated that protease inhibitors also attenuate high blood pressure in HIV patients [30]. However, the role of (P)RR- and renin-mediated proteolytic activity in HIV replication has not been studied. Recently, Hpr has been reported to cause activation of NF-κB and HIV-LTR [31], but the role of (P)RR was not evaluated in these studies. In the present study, TCs silenced for (P)RR displayed attenuated activation of NF-κB in response to HIV. Thus, our data suggest that Hpr also uses the (P)RR signaling cascade for HIV replication in TCs.
HIV has been shown to enhance its replication through activation of NF-κB [32, 33]. In the present study, renin-treated and HIV-infected TCs displayed enhanced activation of NF-κB, which was minimized in the cells lacking renin. Similarly, cells lacking (P)RR displayed an attenuated effect of HIV on TC NF-κB activation. These findings indicate that both (P)RR and renin participate in HIV-induced NF-κB activation. Moreover, VDA inhibits HIV replication, possibly through the upregulation of VDR and down-regulation of renin expression in HIV-infected TCs.
Miyake et al. [34] studied the effect of an NF-κB inhibitor on HIV-1 replication in a human TC line and PHA-stimulated PBMCs. The NF-κB inhibitor attenuated both constitutive NF-κB and HIV-LTR promoter activity in HIV-1-infected PHA-PBMCs. NF-κB inhibition down-regulated integration of HIV-1 provirus into the host genome and decreased HIV-1 expression in the human TC line. The investigators suggested that NF-κB not only regulates early events but also initiates and accelerates the expression of HIV-1.
Patients with HIV infection have been reported to have a 25(OH) D2 (calciferol, vitamin D3) deficiency [35]. However, the cause-and-effect relationship between 25(OH) D2 deficiency and HIV replication is controversial. Because the stability of VDR is dependent on the availability of 1,25(OH)2D (calcitriol, active vitamin D3), its deficiency results in a deficient VDR status. Because 1,25(OH)2D negatively regulates renin [25], patients with HIV infection are likely to have higher levels of renin. Our findings in the present study are consistent with the notion that replenishment of VDA would not only lower renin expression but would also impede HIV replication. In addition, 1,25(OH)2D has been known to inhibit activation of NF-κB in several cell types by multiple mechanisms. In dendritic cells, 1,25(OH)2D directly suppresses RelB transcription [36]. It mitigates an increase in NF-κB p50 and its precursor p105 and c-Rel proteins in activated lymphocytes [37]. Furthermore, it decreases the DNA binding capacity of NF-κB in fibroblasts [38]. Similarly, a VDA attenuates NF-κB p65 nuclear translocation in pancreatic islet cells [39]. 1,25(OH)2D has also been shown to down-regulate HIV replication in macrophages through enhancing autophagy [40]. However, it remains to be determined whether VDR is directly involved in the regulation of the NF-κB pathway.
In summary (Fig. 7), in high-renin states such as HIV infection, renin interacts with (P)RR, which then leads to the release of PLZF from (P)RR and its translocation to the nucleus. PLZF activates the PI3K pathway, leading to the activation of NF-κB and its binding to the LTR promoter site, which in turn generates Gag polyprotein. Gag polyprotein is cleaved by both renin and Hpr and results in the release of proviral proteins, including p24.
ACKNOWLEDGMENTS
This work was supported by grants R01DK084910, R01DK083931, and R01DK098074 to P.C.S. from the U.S. National Institutes of Health, Bethesda, MD, USA.
The authors thank the NIH AIDS Reagent Program for providing the reagents and Novartis for providing the aliskiren.
Footnotes
- Agt
- angiotensinogen
- CD
- cluster of differentiation
- CT
- comparative threshold
- Gag
- group-specific antigen
- Hpr
- HIV protease
- IP
- immunoprecipitation
- LTR
- long terminal repeat
- PI3K
- phosphoinositide 3-kinase
- PLZF
- promyelocytic leukemia zinc finger protein
- p-p65
- phosphorylated protein 65
- (P)RR
- (pro)renin receptor
- RAS
- renin—angiotensin system
- RIPA
- radioimmunoprecipitation assay
- SCR
- scrambled siRNA
- si
- small interfering
- si(P)RR
- siRNA-(P)RR
- siRen
- siRNA-renin
- siVDR
- siRNA-VDR
- Src
- sarcoma
- TC
- T cell
- V-ATPase
- vacuolar type H+-ATPase
- VDR
- vitamin D receptor
- VDA
- vitamin D analogue
AUTHORSHIP
N.C., K.A., X.L., P.R., performed the experiments. J.Mi., M.H., and J.Mc. analyzed the data. A.M. designed the experiments. P.C.S. analyzed the data, designed the experiments, and wrote the manuscript.
DISCLOSURE
The authors declare no conflicts of interest.
REFERENCES
- 1. Nguyen G., Delarue F., Burcklé C., Bouzhir L., Giller T., Sraer J. D. (2002) Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J. Clin. Invest. 109, 1417–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ichihara A. (2012) (Pro) renin receptor and vacuolar H (+)-ATPase. Keio. J. Med. 61, 73–78. [DOI] [PubMed] [Google Scholar]
- 3. Pickering G. (1967) Renin mechanisms and hypertension. Circ. Res. J. 21 (Suppl. 2), 1–2. [PubMed] [Google Scholar]
- 4. Lambers Heerspink H. J., Perkovic V., de Zeeuw D. (2009) Renal and cardio-protective effects of direct renin inhibition: a systematic literature review. J. Hypertens. 27, 2321–2331. [DOI] [PubMed] [Google Scholar]
- 5. Van den Heuvel M., Batenburg W. W., Danser A. H. (2009) Diabetic complications: a role for the prorenin-(pro)renin receptor-TGF-beta1 axis? Mol. Cell. Endocrinol. 302, 213–218. [DOI] [PubMed] [Google Scholar]
- 6. Deinum J., Rønn B., Mathiesen E., Derkx F. M., Hop W. C., Schalekamp M. A. (1999). Increase in serum prorenin precedes onset of microalbuminuria in patients with insulin-dependent diabetes mellitus. Diabetologia 42, 1006–1010. [DOI] [PubMed] [Google Scholar]
- 7. Burcklé C. A., Jan Danser A. H., Müller D. N., Garrelds I. M., Gasc J. M., Popova E., Plehm R., Peters J., Bader M., Nguyen G. (2006) Elevated blood pressure and heart rate in human renin receptor transgenic rats. Hypertension 47, 552–556. [DOI] [PubMed] [Google Scholar]
- 8. Cruciat C. M., Ohkawara B., Acebron S. P., Karaulanov E., Reinhard C., Ingelfinger D., Boutros M., Niehrs C. (2010) Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science 327, 459–463. [DOI] [PubMed] [Google Scholar]
- 9. Schefe J. H., Menk M., Reinemund J., Effertz K., Hobbs R. M., Pandolfi P. P., Ruiz P., Unger T., Funke-Kaiser H. (2006) A novel signal transduction cascade involving direct physical interaction of the renin/prorenin receptor with the transcription factor promyelocytic zinc finger protein. Circ. Res. 99, 1355–1366. [DOI] [PubMed] [Google Scholar]
- 10. Schefe J. H., Unger T., Funke-Kaiser H. (2008) PLZF and the (pro)renin receptor. J. Mol. Med. (Berl.) 86, 623–627. [DOI] [PubMed] [Google Scholar]
- 11. Liu G., Hitomi H., Hosomi N., Shibayama Y., Nakano D., Kiyomoto H., Ma H., Yamaji Y., Kohno M., Ichihara A., Itoh H., Nishiyama A. (2011) Prorenin induces vascular smooth muscle cell proliferation and hypertrophy via epidermal growth factor receptor-mediated extracellular signal-regulated kinase and Akt activation pathway. J. Hypertens. 29, 696–705. [DOI] [PubMed] [Google Scholar]
- 12. Ichihara A., Kaneshiro Y., Takemitsu T., Sakoda M., Suzuki F., Nakagawa T., Nishiyama A., Inagami T., Hayashi M. (2006) Nonproteolytic activation of prorenin contributes to development of cardiac fibrosis in genetic hypertension. Hypertension 47, 894–900. [DOI] [PubMed] [Google Scholar]
- 13. Matavelli L. C., Huang J., Siragy H. M. (2010) (Pro)renin receptor contributes to diabetic nephropathy by enhancing renal inflammation. Clin. Exp. Pharmacol. Physiol. 37, 277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Song R., Yosypiv I. V. (2011) (Pro)renin receptor in kidney development and disease. Int J. Nephrol. 2011, 247048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang J., Matavelli L. C., Siragy H. M. (2011) Renal (pro)renin receptor contributes to development of diabetic kidney disease through transforming growth factor-β1-connective tissue growth factor signalling cascade. Clin. Exp. Pharmacol. Physiol. 38, 215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guzik T. J., Hoch N. E., Brown K. A., McCann L. A., Rahman A., Dikalov S., Goronzy J., Weyand C., Harrison D. G. (2007) Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J. Exp. Med. 204, 2449–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. De Miguel C., Guo C., Lund H., Feng D., Mattson D. L. (2011) Infiltrating T lymphocytes in the kidney increase oxidative stress and participate in the development of hypertension and renal disease. Am. J. Physiol. Renal Physiol. 300, F734–F742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chandel N., Husain M., Goel H., Salhan D., Lan X., Malhotra A., McGowan J., Singhal P. C. (2013) VDR hypermethylation and HIV-induced T cell loss. J. Leukoc. Biol. 93, 623–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chandel N., Sharma B., Husain M., Salhan D., Singh T., Rai P., Mathieson P. W., Saleem M. A., Malhotra A., Singhal PC. (2013) HIV compromises integrity of the podocyte actin cytoskeleton through downregulation of the vitamin D receptor. Am. J. Physiol. Renal Physiol. 304, F1347–F1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Salhan D., Husain M., Subrati A., Goyal R., Singh T., Rai P., Malhotra A., Singhal P. C. (2012) HIV-induced kidney cell injury: role of ROS-induced downregulated vitamin D receptor. Am. J. Physiol. Renal Physiol. 303, F503–F514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Deb D. K., Sun T., Wong K. E., Zhang Z., Ning G., Zhang Y., Kong J., Shi H., Chang A., Li Y. C. (2010) Combined vitamin D analog and AT1 receptor antagonist synergistically block the development of kidney disease in a model of type 2 diabetes. Kidney Int. 77, 1000–1009. [DOI] [PubMed] [Google Scholar]
- 22. Freundlich M., Quiroz Y., Zhang Z., Zhang Y., Bravo Y., Weisinger J. R., Li Y. C., Rodriguez-Iturbe B. (2008) Suppression of renin-angiotensin gene expression in the kidney by paricalcitol. Kidney Int. 74, 1394–1402. [DOI] [PubMed] [Google Scholar]
- 23. Mikulak J., Teichberg S., Faust T., Schmidtmayerova H., Singhal P. C. (2009) HIV-1 harboring renal tubular epithelial cell interaction with T cells results in T cell trans-infection. Virology 385, 105–114. [DOI] [PubMed] [Google Scholar]
- 24. Erickson-Viitanen S., Manfredi J., Viitanen P., Tribe D. E., Tritch R., Hutchison C. A., 3rd, Loeb D. D., Swanstrom R. (1989) Cleavage of HIV-1 gag polyprotein synthesized in vitro: sequential cleavage by the viral protease. AIDS Res. Hum. Retroviruses 5, 577–591. [DOI] [PubMed] [Google Scholar]
- 25. Li Y. C., Kong J., Wei M., Chen Z. F., Liu S. Q., Cao L. P. (2002) 1,25-Dihydroxyvitamin D3 is a negative endocrine regulator of the renin–angiotensin system. J. Clin. Invest. 110, 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chan J.K., Greene W. C. (2012) Dynamic roles for NF-κB in HTLV-I and HIV-1 retroviral pathogenesis. Immunol. Rev. 246, 286–310. [DOI] [PubMed] [Google Scholar]
- 27. DesJarlais R. L., Seibel G. L., Kuntz I. D., Furth P. S., Alvarez J. C., Ortiz de Montellano P. R., DeCamp D. L., Babé L. M., Craik C. S. (1990) Structure-based design of nonpeptide inhibitors specific for the human immunodeficiency virus 1 protease. Proc. Natl. Acad. Sci. U. S. A. 87, 6644–6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sharma S. K., Evans D. B., Hui J. O., Heinrikson R. L. (1991) Could angiotensin I be produced from a renin substrate by the HIV-1 protease? Anal. Biochem. 198, 363–367. [DOI] [PubMed] [Google Scholar]
- 29. Hyland L. J., Meek T. D. (1991) Adaptation of the plasma renin radioimmunoassay for use with HIV-1 protease. Anal. Biochem. 197, 225–230. [DOI] [PubMed] [Google Scholar]
- 30. Tzoupis H., Leonis G., Megariotis G., Supuran C. T., Mavromoustakos T., Papadopoulos M. G. (2012) Dual inhibitors for aspartic proteases HIV-1 PR and renin: advancements in AIDS-hypertension-diabetes linkage via molecular dynamics, inhibition assays, and binding free energy calculations. J. Med. Chem. 55, 5784–5796. [DOI] [PubMed] [Google Scholar]
- 31. Bren G. D., Whitman J., Cummins N., Shepard B., Rizza S. A., Trushin S. A., Badley A. D. (2008) Infected cell killing by HIV-1 protease promotes NF-kappaB dependent HIV-1 replication. PLoS One 3, e2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cullen B. R. (1991) Regulation of HIV-1 gene expression. FASEB J. 5, 2361–2368. [DOI] [PubMed] [Google Scholar]
- 33. Griffin G. E., Leung K., Folks T. M., Kunkel S., Nabel G. J. (1989) Activation of HIV gene expression during monocyte differentiation by induction of NF-kappa B. Nature 339, 70–73. [DOI] [PubMed] [Google Scholar]
- 34. Miyake A., Ishida T., Yamagishi M., Hara T., Umezawa K., Watanabe T., Horie R. (2010) Inhibition of active HIV-1 replication by NF-kappaB inhibitor DHMEQ. Microbes Infect. 12, 400–408. [DOI] [PubMed] [Google Scholar]
- 35. Pinzone M. R., Di Rosa M., Malaguarnera M., Madeddu G., Focà E., Ceccarelli G., d'Ettorre G., Vullo V., Fisichella R., Cacopardo B., Nunnari G. (2013) Vitamin D deficiency in HIV infection: an underestimated and undertreated epidemic. Eur. Rev. Med. Pharmacol. Sci. 17, 1218–1232. [PubMed] [Google Scholar]
- 36. Griffin M. D., Dong X., Kumar R. (2007) Vitamin D receptor-mediated suppression of RelB in antigen presenting cells: a paradigm for ligand-augmented negative transcriptional regulation. Arch. Biochem. Biophys. 460, 218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yu X. P., Bellido T., Manolagas S. C. (1995) Down-regulation of NF-kappa B protein levels in activated human lymphocytes by 1,25-dihydroxyvitamin D3. Proc. Natl. Acad. Sci. U. S. A. 92, 10990–10994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Harant H., Wolff B., Lindley I. J. (1998) 1Alpha,25-dihydroxyvitamin D3 decreases DNA binding of nuclear factor-kappaB in human fibroblasts. FEBS Lett. 436, 329–334. [DOI] [PubMed] [Google Scholar]
- 39. Giarratana N., Penna G., Amuchastegui S., Mariani R., Daniel K. C., Adorini L.A. (2004) vitamin D analog down-regulates proinflammatory chemokine production by pancreatic islets inhibiting T cell recruitment and type 1 diabetes development. J. Immunol. 173, 2280–2287. [DOI] [PubMed] [Google Scholar]
- 40. Campbell G. R., Spector S. A. (2011) Hormonally active vitamin D3 (1alpha,25-dihydroxycholecalciferol) triggers autophagy in human macrophages that inhibits HIV-1 infection. J. Biol. Chem. 286, 18890–18902. [DOI] [PMC free article] [PubMed] [Google Scholar]