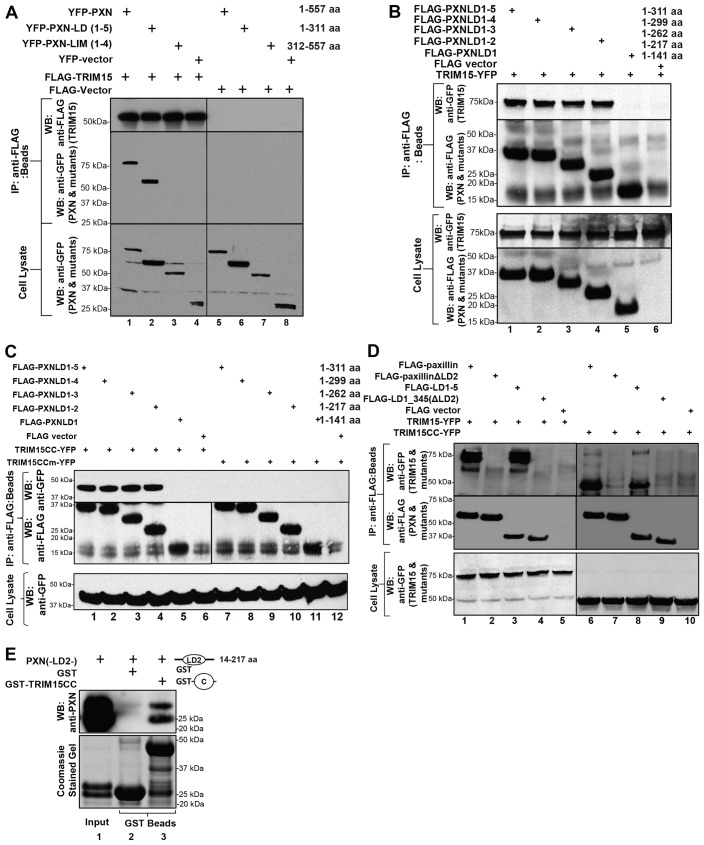
Fig. 4.
TRIM15 interacts with the LD2 motif of paxillin. (A) TRIM15 interacts with the N-terminal LD-containing fragment of paxillin. Lysates from HEK293 cells coexpressing YFP-tagged wild-type or mutant paxillin (PXN) or empty vector together with either FLAG–TRIM15 (lanes 1–4) or empty FLAG vector control (lanes 5–8) were immunoprecipitated using antibodies against the FLAG epitope. The immunoprecipitates (IP, beads) and cell lysates were analyzed by western blotting (WB) using antibodies against GFP or FLAG as indicated. (B–D) Paxillin LD2 is required for an interaction with TRIM15. HEK293 cell lysates coexpressing the indicated wild-type or mutant FLAG-tagged paxillin variants or empty vector control together with full-length TRIM15–YFP, the coiled-coil domain (TRIM15CC–YFP) or the mutated coiled-coil domain (V213G, L1216R; TRIM15CCm–YFP) were immunoprecipitated and processed as described in A. The amino acid coordinates for paxillin deletions are also indicated. (E) Paxillin LD2 motif binds directly to TRIM15 coiled-coil domain in vitro. Paxillin LD2 [PXN(-LD2-)] protein (input) was incubated with glutathione-S-transferase (GST) fusion proteins of the TRIM15 coiled-coil domain (GST–TRIM15CC). GST was used as the specificity control. The co-precipitated LD2 protein was detected by western blotting using polyclonal anti-paxillin antibodies that can also recognize the LD2 region of paxillin (upper panel). A Coomassie-Blue-stained gel with the input LD2 and GST fusion proteins is shown below as a reference.