Abstract
Purpose
To evaluate the efficacy of 2 dexamethasone intravitreal implants and 1 ranibizumab intravitreal injection after a bilateral postoperative complication of cataract surgery as pseudophakic cystoid macular edema.
Patients and Methods
A 70-year-old male patient with systemic hypertension developed a progressive cystoid macular edema (CME) in both eyes starting between 10 and 20 days after cataract surgery. Two intravitreal dexamethasone implants and 1 ranibizumab injection were administered; first in the right eye (RE) and then in the left eye (LE). The patient was checked for 1 whole week and then once a month for 5 months after the injections.
Results
One month after the first dexamethasone implant in his RE, the spectral domain optical coherence tomography (SD-OCT) showed a progressive reduction of the foveal thickness until a complete resolution of the CME occurred, which was associated with an improvement of visual acuity. After 3 months, the SD-OCT showed a relapse of the CME, which was then treated with 1 injection of ranibizumab. One month after this injection, there was a complete resolution of the CME. A new CME in his RE was diagnosed 2 months after the last ranibizumab injection; it was treated with a new dexamethasone implant. A complete resolution of the CME was obtained; a normal foveal profile was still present 5 months after the last injection, and the best-corrected visual acuity was 20/20. His LE developed a CME 40 days after surgery. One intravitreal injection of ranibizumab was first administered in his LE, with a complete resolution of the CME at SD-OCT 2 weeks later. As observed in his RE, 40 days after the ranibizumab injection, there was a relapse of the CME that was treated with 1 intravitreal injection of dexamethasone implant. Five months later, the patient showed a worsening of the CME, but it was completely resolved with a second dexamethasone injection. After 3 months, the foveal thickness was back to normal with a BCVA of 20/20.
Conclusion
Treatment with dexamethasone implants (Ozurdex®) and ranibizumab injections (Lucentis®) induced a progressive reduction of our patient's CME after cataract surgery (Irvine-Gass syndrome) until a complete normal foveal thickness was restored and his visual function was improved despite the order of injections.
Key words: Irvine-Gass syndrome, Cystoid macular edema, Intravitreal injection, Dexamethasone, Ranibizumab, Cataract, Surgery, Complication
Introduction
Pseudophakic cystoid macular edema (PCME), also called Irvine-Gass syndrome, is a common cause of visual impairment after cataract surgery. The occurrence of PCME has declined with the use of modern surgical techniques [1]. In fact, the innovations in instrumentation, lens design and surgical technique have led to positive outcomes following cataract surgery. Nevertheless, it is possible that cystoid macular edema (CME) develops after modern cataract surgery, and this can result in a significant loss of visual acuity [2].
The pathogenesis of Irvine-Gass syndrome still remains undefined today. Epidemiological studies underlined that this syndrome can most frequently occur after a complicated surgery or in patients with ocular diseases such as diabetic retinopathy or uveitis [3]. The diagnosis is clinical and can be confirmed by fluorescein angiography and spectral domain optical coherence tomography (SD-OCT).
Case Presentation
A 70-year-old male patient visited our department complaining of a decrease of visual acuity about 10–20 days after surgery, which was performed in October 2012 on his right eye (RE), and in March 2013 on his left eye (LE). The surgery consisted of a bilateral cataract phacoemulsification with a lens implant in the bag; it was done without any surgical complications. The diagnosis of bilateral Irvine-Gass syndrome was clinically made by performing an ophthalmoscopic slit-lamp biomicroscopy and by SD-OCT (Cross Line, MM5, 3D Macular, RTVue® SD-OCT) which showed a cystoid macular edema (CME).
Clinical History of the RE
One day after his cataract phacoemulsification surgery with an intraocular lens implant, the postoperative follow-up showed a best-corrected visual acuity (BCVA) of 20/20. At the slit-lamp examination, the anterior segment was normal and with no signs of any ocular inflammation. His intraocular pressure (IOP) at Goldmann tonometry and iCare [4] was 17 mm Hg.
Ten days after surgery, the patient complained of a decrease in visual acuity (BCVA 20/40) and of floating vision that was confirmed by a positive Amsler test. His medical therapy was started with topical, nonsteroidal anti-inflammatory drugs (NSAIDs) 4 times/day and with indomethacin tablets of 50 mg per os 3 times/day for 15 days. This therapy had no effect in terms of improving his BCVA or CME at SD-OCT. Fourteen days later, the patient was subsequently treated with 3 peribulbar methylprednisolone injections (1/week) that did not produce any positive clinical effects, and his BCVA decreased to 20/70.
Fifty-nine days after the phacoemulsification surgery, his RE was treated with an intravitreal injection [5] of dexamethasone Ozurdex® (Allergan, Inc., Irvine, Calif., USA). Ozurdex is a biodegradable copolymer of lactic- and glycolic-acid containing micronized dexamethasone, which forms a matrix structure that is able to gradually release the total dose over time (after the injection) [6]. The most common local side effect can be an increase of IOP and cataract [7].
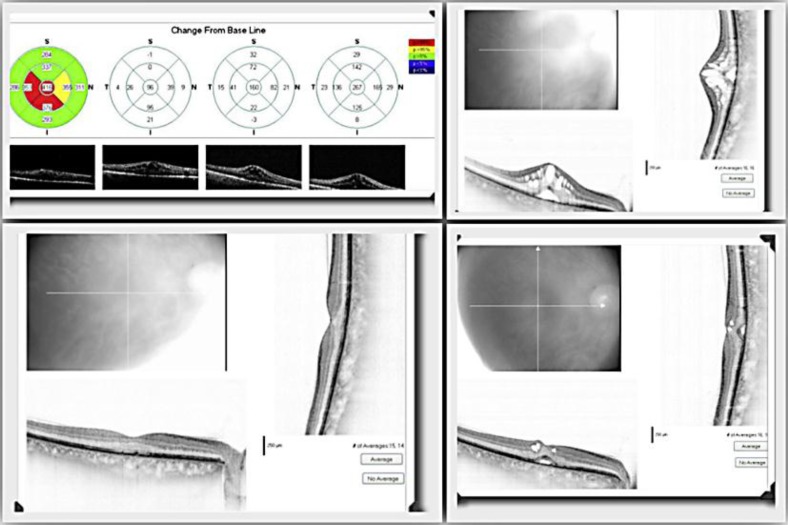
Seven days after the injection, SD-OCT of his RE showed a significant reduction of foveal thickness (369 μm), together with an improvement of BCVA (20/25) and a stable IOP (17 mm Hg). Fifty days after the injection, SD-OCT showed a complete resolution of his CME with a normalization of the foveal profile and an improvement of his BCVA which returned to 20/20. The IOP remained stable at 17 mm Hg (fig. 1).
Fig. 1.
Progression of CME in the RE before and after using Ozurdex.
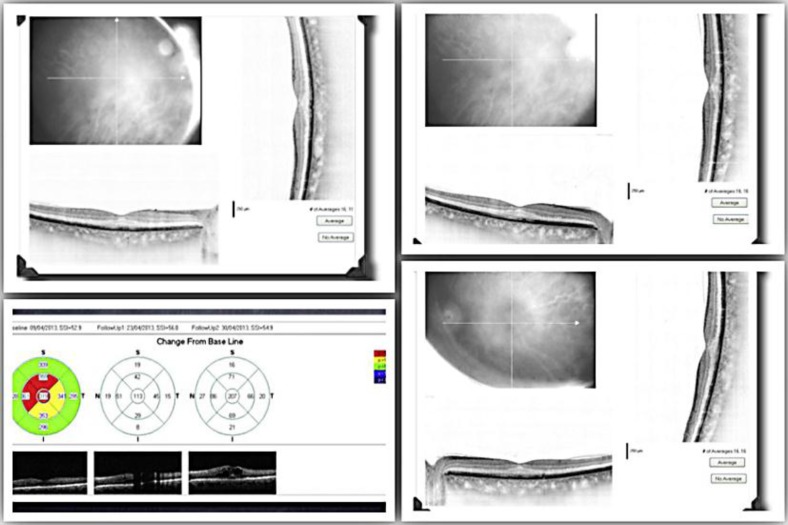
Eighty-four days after the Ozurdex® injection, the patient presented with a relapse of CME in his RE, shown on SD-OCT (fig. 2), associated with loss of visual acuity (BCVA 20/30). At this time, his RE was treated with an injection of ranibizumab Lucentis® (NovartisPharma AG, Basel, Switzerland, and Genentech Inc., South San Francisco, Calif., USA). Ranibizumab is a recombinant, humanized monoclonal antibody antigen-binding fragment directed against vascular endothelial growth factor (VEGF)-A. VEGF-A belongs to a family of mitogenic glycoproteins that promote angiogenesis by activating the cell surface VEGF receptors, present in endothelial and mural cells, via a tyrosine kinase signaling pathway. Ranibizumab acts against all VEGF isoforms of VEGF-A [8]. Six days after this therapy there was a complete resolution of CME with a BCVA of 20/20.
Fig. 2.
Relapse of CME 3 months after Ozurdex.
Two months after the ranibizumab injection, a new relapse of CME was clinically diagnosed with SD-OCT. It was associated with a loss of visual acuity (20/50 BCVA). This new complication was treated with a second implant of a dexamethasone injection Ozurdex. Five months later, a complete and definitive resolution of the patient's pseudophakic CME (PCME), with a BCVA of 20/20, was found. For this result, the patient decided to do surgery in his LE.
Clinical History of the LE
One day after his cataract phacoemulsification surgery with intraocular lens implant, the postoperative control showed a BCVA of 20/20. At the slit lamp examination, the anterior segment was normal, with no signs of ocular inflammation. His IOP at Goldmann tonometry was 16 mm Hg.
Twenty days after cataract surgery, the LE developed CME, diagnosed by SD-OCT, that was not associated with a decrease of his BCVA (20/20). The same medical therapy for his RE, with topical NSAIDs and Indomethacin 50 mg per os, was performed for 15 days, but without any clinically relevant results.
Forty-one days after cataract surgery, a worsening of his CME associated with a progressive decrease of the patient's BCVA (20/50) was present, and this time the LE was first treated with an intravitreal injection of ranibizumab (Lucentis). Ranibizumab was chosen as a first injection in order to test if a different sequence in therapy with intravitreal injections in the RE could be associated to a better and prolonged improvement of his visual acuity and the macular thickness.
Thirteen days after the ranibizumab injection, SD-OCT of the LE showed a significant reduction of foveal thickness (354 μm) related to an improvement of BCVA that was 20/25. IOP was 16 mm Hg.
Forty-one days after the injection of ranibizumab, the patient showed a relapse of CME in his LE, shown on SD-OCT, and associated with visual loss acuity that was 20/40. The LE was then treated with an injection of dexamethasone Ozurdex, and 7 days after the therapy, the patient showed a partial resolution of CME with an improvement of his visual acuity to 20/25. There was no change of IOP (16 mm Hg). Thirty and 80 days after the injection of dexamethasone in his LE, SD-OCT showed a complete progressive resolution of CME, and the BCVA improved to 20/20.
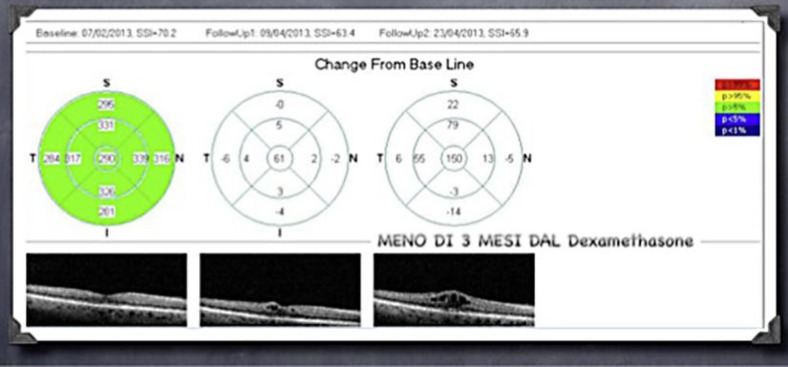
Five months after dexamethasone therapy, a new relapse of CME associated with visual loss (20/30 BCVA) was observed. His LE was treated with a second implant of Ozurdex, obtaining a complete and definitive resolution of his PCME and an improvement of his BCVA to 20/20 (fig. 3).
Fig. 3.
Complete and definitive resolution of PCME.
Discussion
Irvine-Gass syndrome was described for the first time in 1953 by Irvine [9] as a CME that specifically arose after cataract surgery. Gass and Norton [10] subsequently studied the characteristics of this new disease with fluorescein angiography. Epidemiological studies show that the Irvine-Gass syndrome tends to have a spontaneous remission in 70% of the cases; 50% of these cases come to a resolution within 6 months after cataract surgery, and 90% in 2 years [11]. Because of its positive resolution, in most of the cases, there is no standardized treatment protocol for Irvine-Gass syndrome.
The way this syndrome is handled is based on its inflammatory pathogenesis. Over the years, several types of treatment have been proposed by a topical, periocular or an intravitreal use of corticosteroids. In 2003, Benhamou et al. [12] reported the use of 8 mg of intravitreal triamcinolone to treat 3 cases of refractory chronic pseudophakic CME. It showed a reduction of macular thickness and an improvement of visual acuity. Nevertheless, the effects were transient. Other authors subsequently obtained controversial results with the use of anti-VEGF in the presence of persistent CME [13]. In the United States of America, the FDA recently approved the use of Ozurdex for the treatment of CME not only associated with retinal vein occlusion, such as in post-noninfectious posterior uveitis [14], but also in the treatment of persistent macular edema resulting from uveitis or PCME [15].
In our study, the intravitreal therapy of PCME has proven to be effective for both, through the use of anti-VEGF Lucentis, and of dexamethasone (Ozurdex) in order to obtain the reduction of CME and the normalization of the foveal thickness. Prolonged activity in terms of resolution of edema by Ozurdex compared to Lucentis lead to the conclusion that the use of an intravitreal injection of dexamethasone can be considered a good therapeutic choice for Irvine-Gass syndrome, which is resistant to other possible treatments, and it may be considered in further studies with a large number of patients.
References
- 1.Yonekawa Y, Kim IK. Pseudophakic cystoid macular edema. Curr Opin Ophthalmol. 2012;23:26–32. doi: 10.1097/ICU.0b013e32834cd5f8. [DOI] [PubMed] [Google Scholar]
- 2.Mohammadpour M, Jafarinasab MR, Javadi MA. Outcomes of acute postoperative inflammation after cataract surgery. Eur J Ophthalmol. 2007;17:20–28. doi: 10.1177/112067210701700104. [DOI] [PubMed] [Google Scholar]
- 3.Nelson ML, Martidis A. Managing cystoid macular edema after cataract surgery. Curr Opin Ophthalmol. 2003;14:39–43. doi: 10.1097/00055735-200302000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Scuderi GL, Cascone NC, Regine F, Perdicchi A, Cerulli A, Recupero SM. Validity and limits of the rebound tonometer (ICare®): clinical study. Eur J Ophthalmol. 2011;21:251–257. doi: 10.5301/EJO.2010.3712. [DOI] [PubMed] [Google Scholar]
- 5.Lambiase A, Abdolrahimzadeh S, Recupero SM. An update on intravitreal implants in use for eye disorders. Drugs Today (Barc) 2014;50:239–249. doi: 10.1358/dot.2014.50.3.2103755. [DOI] [PubMed] [Google Scholar]
- 6.Querques L, Querques G, Lattanzio R, Gigante SR, Del Turco C, Corradetti G, Cascavilla ML, Bandello F. Repeated intravitreal dexamethasone implant (Ozurdex®) for retinal vein occlusion. Ophthalmologica. 2013;229:21–25. doi: 10.1159/000342160. [DOI] [PubMed] [Google Scholar]
- 7.London NJ, Chiang A, Haller JA. The dexamethasone drug delivery system: indications and evidence. Adv Ther. 2011;28:351–366. doi: 10.1007/s12325-011-0019-z. [DOI] [PubMed] [Google Scholar]
- 8.Fong AH, Lai TY. Long-term effectiveness of ranibizumab for age-related macular degeneration and diabetic macular edema. Clin Interv Aging. 2013;8:467–483. doi: 10.2147/CIA.S36811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irvine SR. A newly defined vitreous syndrome following cataract surgery. Am J Ophthalmol. 1953;36:599–619. doi: 10.1016/0002-9394(53)90302-x. [DOI] [PubMed] [Google Scholar]
- 10.Gass JDM, Norton EWD. Cystoid macular edema and papilledema following cataract extraction: a fluorescein fundoscopic and angiographic study. 1966. Retina. 2003;23:646–661. [PubMed] [Google Scholar]
- 11.Benitah NR, Arroyo JG. Pseudophakic cystoid macular edema. Int Ophthalmol Clin. 2010;50:139–153. doi: 10.1097/IIO.0b013e3181c551da. [DOI] [PubMed] [Google Scholar]
- 12.Benhamou N, Massin P, Haouchine B, et al. Intravitreal triamcinolone for refractory pseudophakic macular edema. Am J Ophthalmol. 2003;135:246–249. doi: 10.1016/s0002-9394(02)01938-4. [DOI] [PubMed] [Google Scholar]
- 13.Arevalo JF, Maia M, Garcia-Amaris RA, et al. Intravitreal bevacizumab for refractory pseudophakic cystoid macular edema: the Pan-American Collaborative Retina Study Group results. Ophthalmology. 2009;116:1481–1487. doi: 10.1016/j.ophtha.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 14.United States Food and Drug Administration. Label and approval history. http://www.accessdata.fda.gov/Scripts/cder/DrugsatFDA/index.cfm. Accessed 11/13/2011.
- 15.Williams GA, Haller JA, Kuppermann BD, et al. Dexamethasone posterior-segment drug delivery system in the treatment of macular edema resulting from uveitis or Irvine-Gass syndrome. Am J Ophthalmol. 2009;147:1048–1054. doi: 10.1016/j.ajo.2008.12.033. [DOI] [PubMed] [Google Scholar]