To the Editor
Pediatric acute myeloid leukemia (AML) is a heterogeneous malignant disease, characterized by abnormal proliferation and impaired differentiation of myeloid cells. Acquired cytogenetic aberrations such as t(8;21)(q22;q22)/AML-ETO, inv(16)(p13q22)/CBFβ-MYH11, t(15;17)(q22;q21)/PML-RARα, rearrangements involving MLL, and molecular aberrations in genes such as FLT3, NPM1, CEBPA, and WT1 are involved in AML pathogenesis and are prognostic factors for treatment outcome.
Recently, constitutional loss-of-function mutations in telomerase complex genes have been implicated as risk factors for AML in adults.1 The telomerase complex is expressed in highly proliferative cells, and is responsible for maintaining telomeres, which cap the ends of chromosomes and protect genomic DNA from eroding during cell divisions. Impaired telomerase function can result in extremely short telomeres, limiting the proliferative capacity of progenitor cells, and may lead to chromosomal instability, thus predisposing to malignant transformation.2 This pathogenesis is manifested in dyskeratosis congenita, an inherited bone marrow (BM) failure syndrome in which patients are at increased risk for malignancies, including AML, and in which telomerase complex mutations are etiologic.2
To date, the significance of telomerase complex mutations as risk factor for AML in children is unknown. Furthermore, although extremely short telomeres in leukemic cells of adult AML patients have been reported to be associated with cytogenetic aberrations and complex karyotype, the clinical implications of these short telomeres are unknown.3–4 In the current study, we assessed the frequency of mutations in the telomerase complex genes TERT, telomerase reverse transcriptase, and TERC, the RNA template of the telomerase complex, in pediatric AML. Furthermore, we measured telomere length in pediatric AML patients at diagnosis and determined its relationship with clinical and molecular characteristics and outcome.
The frequency of mutations in TERT and TERC was determined as previously described1 in diagnostic BM or peripheral blood (PB) samples obtained from a representative cohort (Table S1) of 168 de novo pediatric AML cases, who were enrolled in consecutive DCOG, AML-BFM, and LAME treatment protocols between 1987 and 2008. Samples contained, after enrichment, 80% or more leukemic cells. No mutations in TERC were detected. In TERT, three non-synonymous variants were present in a total of 8 patients (carrier frequency 0.048). In one patient, a novel heterozygous variant in a well-conserved amino acid in exon 2 in the N-terminal extension of TERT, E327D (mRNA NM_198253.2: 1039G/T) was detected. Telomerase activity of this variant, as determined in reconstitution assays in telomerase-deficient VA13 cells,1 was similar to wild-type TERT (94.7±3.5% (mean ± SD)). Furthermore, a heterozygous variant in exon 6 in the reverse transcriptase domain of TERT, T726M (mRNA NM_198253.2: 2235C/T), was found in another patient. T726M, located in the reverse transcriptase domain of telomerase, was not associated with reduced telomerase activity.5 Both mutations were absent among 406 Dutch adult blood donors. Remission material to confirm germ-line origin of these mutations was not available in the two patients. Six of 168 patients carried a heterozygous variant in the C-terminal extension of TERT, A1062T (mRNA NM_198253.2: 3242G/A; carrier frequency 0.036). A1062T was also the most common gene variant in adult AML (n=594; minor allele frequency (MAF) 0.019), with a frequency three times higher than in ethnicity and/or geographically matched controls (n=1110; MAF 0.006).1 In contrast, A1062T was present in a similar carrier frequency in pediatric AML patients (0.048) as in 406 Dutch controls (0.049), composed of two independent cohorts (6/192 (0.031) and 14/214 (0.065)). In three of the six patients carrying A1062T, remission material (<1% blasts by flow cytometry immunophenotyping) was available, confirming germ-line origin of the variant. Telomerase activity of A1062T was reported normal6 or decreased to 60% of wild-type.1 Clinical characteristics of patients carrying TERT variants are described in Table S2. Other variants, all reported in healthy controls and not associated with reduced telomerase function,5 were present in this cohort and are described in Table S3. MAFs of two synonymous variants and variant A279T in patients were similar in controls, demonstrating comparability of both cohorts (Table S3).
Our data are in contrast to adult AML patients, in whom constitutional TERT mutations appear to be a risk factor for AML.1 Although variants E327D and T726M were absent in healthy controls, the relationship of these variants to the etiology of AML is dubious, as the variants showed normal telomerase function when assayed in vitro, karyotype was normal in both patients, and telomere lengths of leukemic cells were within the third quartile of the total cohort. Furthermore, the most common variant in our cohort, A1062T, was present in a comparable frequency in geographically matched controls, and thus, at least in our pediatric AML cohort, it cannot be regarded as a pathologic variant. The carrier frequency of A1062T (0.049) in Dutch blood bank donors is remarkably higher than the MAF of 0.006 in controls in the adult AML study,1 but comparable to a carrier frequency of 0.036 in 600 German controls7 and 0.031 in 477 white controls from the NCI PLCO Cancer Screening Trial.8 This might suggest that in individuals of Caucasian descent, or within specific ethnic subpopulations, the A1062T variant may be less pathogenic than previously thought.
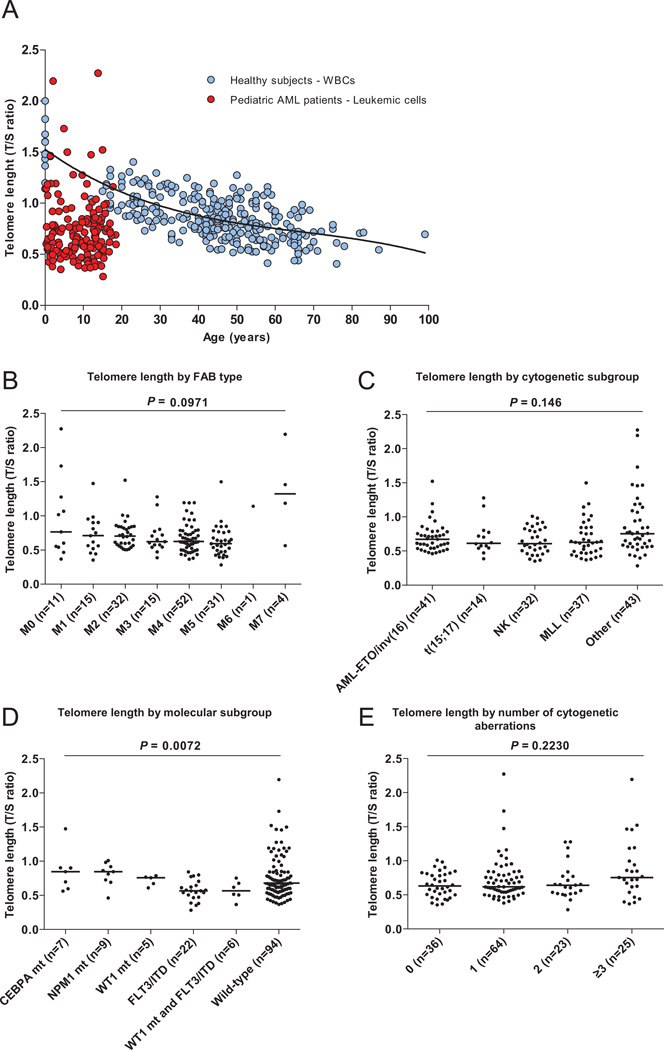
Although mutations in the telomerase complex genes TERT and TERC are infrequent events in pediatric AML, we investigated whether short telomeres in leukemic cells are associated with AML characteristics or contribute to an adverse outcome. Telomere length was successfully determined by qPCR as previously described8 with some modifications in leukemic cells derived from 167 pediatric AML patients at diagnosis. Median telomere length (T/S ratio) was 0.65 (range, 0.28–2.3). When compared to healthy control peripheral blood mononuclear cells, telomere length in leukemic cells was very short (Figure 1A), confirming previous reports.3–4, 9 Because age at diagnosis was not associated with telomere length (Figure 1A), patients were categorized into quartiles based on telomere length without age-adjustment (Table 1). There was no difference in distribution of sex or WBC count among the quartiles. When evaluated as a continuous variable, there was no difference in telomere length among FAB types, nor among AML characteristic cytogenetic subgroups (Figures 1B and 1C). Telomere lengths between prognostic relevant molecular subgroups, determined as described,10 differed significantly (P=0.0072) (Figure 1D). Specifically, cells carrying FLT3/ITD or a WT1 mutation and FLT3/ITD had shorter telomeres than did CEBPA double mutants (P=0.0064 and P=0.051, respectively) and NPM1 mutants (P=0.0012 and P=0.0176, respectively). Cells carrying FLT3/ITD and an NPM1 mutation had telomere lengths similar to cells carrying only FLT3/ITD (n=4; not shown). The significantly shorter telomeres in cells carrying the high-risk molecular aberrations FLT3/ITD and FLT3/ITD with a WT1 mutation might reflect a more extended mitotic history of leukemic cells harboring FLT3/ITD as compared to cells carrying favorable prognostic aberrations. T/S ratio in FLT3/TKD mutant cases (n=3; mean: 0.59, range: 0.43–0.80) was similar to cells carrying FLT3/ITD.
Figure 1. Leukemic cell telomere length and telomere length in biological subgroups.
(A) Telomere length, expressed as telomere to single copy gene ratio (T/S), was determined in leukemic cells derived from 167 pediatric AML patients at diagnosis and total leukocytes derived from 298 healthy NIH blood bank donors, and expressed as a function of age (years). Telomere length in leukemic cells is not associated with age (slope −0.0043 ± 0.0045, r2 0.005, P=0.349), whereas telomere length in total leukocytes decreases as a function of age. The coefficient of variation (CV) in 51 pediatric AML samples that were measured in two independent runs, calculated as mean of the standard deviation (SD) per repeated measurement divided by the mean of all measurements, was 12%. (B) Telomere length by FAB type. Telomere length was not associated with FAB type (median T/S ratio M0: 0.77; M1: 0.71; M2 0.71; M3: 0.62: M4: 0.63; M5: 0.59; M6: 1.14; M7: 1.32; median T/S ratio of complete cohort: 0.65). FAB M1-2 (n=1; T/S ratio 0.38) and unknown FAB types (n=5; T/S ratio 1.09) are not shown. (C) Telomere length by cytogenetic subgroup. Telomere length was not associated with cytogenetic subgroup (median T/S ratio MLL rearranged cases: 0.63; AML-ETO/inv(16): 0.67; t(15;17): 0.61; NK: 0.61; other: 0.75; median T/S ratio of complete cohort: 0.65). (D) Telomere length by molecular subgroup. Patients carrying more than one of the screened mutations are excluded; patients carrying both FLT3/ITD and a WT1 mutation are only included in the WT1 and FLT3/ITD group. Patients carrying a FLT3/TKD mutation are excluded. Telomere length was associated with molecular subgroup (median T/S ratio CEBPA double mutants: 0.85, range: 0.56–1.5; NPM1 mutants: 0.85, range, 0.46–1.0; WT1 mutants: 0.76, range: 0.61–0.79; FLT3/ITD: 0.57, range: 0.28–0.84; WT1 and FLT3/ITD: 0.57, range, 0.37–0.75; wild-type (not carrying any of the former aberrations): 0.67, range: 0.43–0.80). (E) Telomere length by number of cytogenetic aberrations. Nineteen patients, for whom complete karyotypes were not available, are excluded. Telomere length was not associated with the number of cytogenetic aberrations (median T/S ratio 0 aberrations: 0.63; 1 aberration: 0.62; 2 aberrations: 0.64; ≥3 aberrations: 0.76).
Table 1.
Patient characteristics by telomere length quartile.
| Telomere length quartile | ||||||
|---|---|---|---|---|---|---|
| Characteristic | All patients | 1 | 2 | 3 | 4 | P-value |
| Telomere length range, T/S ratio | 0.283–2.27 | 0.283–0.541 | 0.541–0.651 | 0.651–0.838 | 0.838–2.27 | |
| Patients, No. | 167 | 42 | 42 | 42 | 41 | |
| Sex, No. (%) | ||||||
| Male | 92 (55.1) | 26 (61.9) | 20 (47.6) | 24 (57.1) | 22 (53.7) | 0.606a |
| Female | 75 (44.9) | 16 (38.1) | 22 (52.4) | 18 (42.9) | 19 (46.3) | |
| Age, median, y [SD] | 9.7 [5.3] | 9.1 [4.8] | 9.7 [5.4] | 10.4 [5.5] | 9.7 [5.5] | 0.715a |
| Age < 10 y, No. | 86 | 23 | 22 | 20 | 21 | 0.930b |
| Age > 10 y, No. | 81 | 19 | 20 | 22 | 20 | |
| WBC, median (× 109/uL) [SD]* | 47.3 [83.4] | 58.1 [111.4] | 43.1 [55.4] | 33 [82.1] | 40.2 [64.5] | 0.154a |
Abbreviations: mt, mutant; wt, wild-type; WBC, white blood cell count
When evaluated as continuous variable by linear regression analysis, telomere length was associated with WBC (P=0.033, r=−0.175). Telomere length was not associated with leukemic cell count after enrichment.
Kruskall-Wallis test,
Chi-square test
Telomere length was not associated with the number of cytogenetic aberrations (numerical and/or structural), including complex karyotype, defined as three or more cytogenetic aberrations (Figure 1E). These findings are in contrast to adult AML, where patients with an aberrant karyotype had significantly shorter leukemic cell telomeres than did normal karyotype patients,3–4 suggesting that short telomeres in AML might induce chromosomal instability. A limitation of our approach is that we studied average leukemic cell telomere length; however, the shortest telomere per cell seems to determine chromosomal stability.11 Future studies might benefit from single telomere length analysis, in which telomere lengths and fusion events at specific chromosomes can be determined.12
To gain insight into mechanisms that might contribute to the differences in telomere length in AML blast cells, expression levels, determined by microarray analysis (in 157/168 patients; HGU133 Plus 2.0 microarray, Affymetrix)13 of telomere biology-related genes were correlated with telomere length. Expression levels of TERF1, TERF2, TINF2, and POT1 (components of the shelterin complex); WDR79 or TCAB1 (facilitating trafficking of telomerase to Cajal bodies) and TERT, DKC1, NHP2, NOP10, and GAR1 (components of the telomerase complex), did not correlate with telomere length (Table S4).
To assess the influence of telomere length on clinical outcome, telomere length quartiles 2 through 4 were grouped and compared with telomere length quartile 1. Five-year pOS in quartile 1 versus quartiles 2–4 (50±8% versus 61±5%), pEFS (40±8% versus 44±5%), and cumulative incidence of relapse and non-response (50±8% versus 44±5%) were not significantly different (Figure S1). That short telomeres of leukemic cells do not correlate with unfavorable outcome is in accordance with a smaller study in adult AML,4 but in contrast to studies in B-CLL and CML.14–15
To summarize, in pediatric AML, variants in the telomerase complex genes TERT and TERC do not seem to be a risk factor for developing AML. Clinically, loss-of-function mutations in TERC or TERT might increase the risk of chemotherapy-related toxicity and are relevant in selecting sibling donors for hematopoietic stem cell transplantation. However, given the results of this study, in the absence of classical features of dyskeratosis congenita, or a family history positive for BM failure, liver cirrhosis, or pulmonary fibrosis, screening for mutations in TERT or TERC in sporadic AML cases does not seem relevant. Short telomeres correlate with the high-risk molecular aberration FLT3/ITD, but survival is not significantly influenced by telomere length in this pediatric AML cohort.
Acknowledgements
AMA was supported by the KiKa Foundation, Amstelveen, The Netherlands, the René Vogels Foundation, Oirschot, and Erasmus Trustfonds, Rotterdam, The Netherlands. This research was supported in part by the NIH (NHLBI) Intramural Research Program.
Footnotes
Authorship contributions
AMA, RTC, SK, NSY, CMZ, RP, VHJV, MHE conceived and designed the experiments; AMA, SK performed the experiments; AMA, RTC, CMZ, CW, EC, MZ, RP, VHJV, MHE analyzed the data; AMA, RTC, NSY, CMZ, CW, SK, EC, AB, KG, GJLK, VH, TWK, DR, JT, MZ, RP, VHJV, MHE contributed reagents, materials and analysis tools and approved the final version of the paper; AMA, RTC, NSY, VHJV, MHE wrote the paper.
Disclosure of conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1.Calado RT, Regal JA, Hills M, Yewdell WT, Dalmazzo LF, Zago MA, et al. Constitutional hypomorphic telomerase mutations in patients with acute myeloid leukemia. Proc Natl Acad Sci U S A. 2009;106:1187–1192. doi: 10.1073/pnas.0807057106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calado RT, Young NS. Telomere diseases. N Engl J Med. 2009;361:2353–2365. doi: 10.1056/NEJMra0903373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swiggers SJ, Kuijpers MA, de Cort MJ, Beverloo HB, Zijlmans JM. Critically short telomeres in acute myeloid leukemia with loss or gain of parts of chromosomes. Genes Chromosomes Cancer. 2006;45:247–256. doi: 10.1002/gcc.20286. [DOI] [PubMed] [Google Scholar]
- 4.Hartmann U, Brummendorf TH, Balabanov S, Thiede C, Illme T, Schaich M. Telomere length and hTERT expression in patients with acute myeloid leukemia correlates with chromosomal abnormalities. Haematologica. 2005;90:307–316. [PubMed] [Google Scholar]
- 5.Xin ZT, Beauchamp AD, Calado RT, Bradford JW, Regal JA, Shenoy A, et al. Functional characterization of natural telomerase mutations found in patients with hematologic disorders. Blood. 2007;109:524–532. doi: 10.1182/blood-2006-07-035089. [DOI] [PubMed] [Google Scholar]
- 6.Alder JK, Chen JJ, Lancaster L, Danoff S, Su SC, Cogan JD, et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci U S A. 2008;105:13051–13056. doi: 10.1073/pnas.0804280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartmann D, Srivastava U, Thaler M, Kleinhans KN, N'Kontchou G, Scheffold A, et al. Telomerase gene mutations are associated with cirrhosis formation. Hepatology. 2011;53:1608–1617. doi: 10.1002/hep.24217. [DOI] [PubMed] [Google Scholar]
- 8.Calado RT, Brudno J, Mehta P, Kovacs JJ, Wu C, Zago MA, et al. Constitutional telomerase mutations are genetic risk factors for cirrhosis. Hepatology. 2011;53:1600–1607. doi: 10.1002/hep.24173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamada O, Oshimi K, Motoji T, Mizoguchi H. Telomeric DNA in normal and leukemic blood cells. J Clin Invest. 1995;95:1117–1123. doi: 10.1172/JCI117759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balgobind BV, Hollink IH, Arentsen-Peters ST, Zimmermann M, Harbott J, Beverloo HB, et al. Integrative analysis of type-I and type-II aberrations underscores the genetic heterogeneity of pediatric acute myeloid leukemia. Haematologica. 2011;96:1478–1487. doi: 10.3324/haematol.2010.038976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hemann MT, Strong MA, Hao LY, Greider CW. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell. 2001;107:67–77. doi: 10.1016/s0092-8674(01)00504-9. [DOI] [PubMed] [Google Scholar]
- 12.Baird DM, Rowson J, Wynford-Thomas D, Kipling D. Extensive allelic variation and ultrashort telomeres in senescent human cells. Nat Genet. 2003;33:203–207. doi: 10.1038/ng1084. [DOI] [PubMed] [Google Scholar]
- 13.Hollink IH, van den Heuvel-Eibrink MM, Arentsen-Peters ST, Pratcorona M, Abbas S, Kuipers JE, et al. NUP98/NSD1 characterizes a novel poor prognostic group in acute myeloid leukemia with a distinct HOX gene expression pattern. Blood. 2011;118:3645–3656. doi: 10.1182/blood-2011-04-346643. [DOI] [PubMed] [Google Scholar]
- 14.Roos G, Krober A, Grabowski P, Kienle D, Buhler A, Dohner H, et al. Short telomeres are associated with genetic complexity, high-risk genomic aberrations, and short survival in chronic lymphocytic leukemia. Blood. 2008;111:2246–2252. doi: 10.1182/blood-2007-05-092759. [DOI] [PubMed] [Google Scholar]
- 15.Brummendorf TH, Holyoake TL, Rufer N, Barnett MJ, Schulzer M, Eaves CJ, et al. Prognostic implications of differences in telomere length between normal and malignant cells from patients with chronic myeloid leukemia measured by flow cytometry. Blood. 2000;95:1883–1890. [PubMed] [Google Scholar]