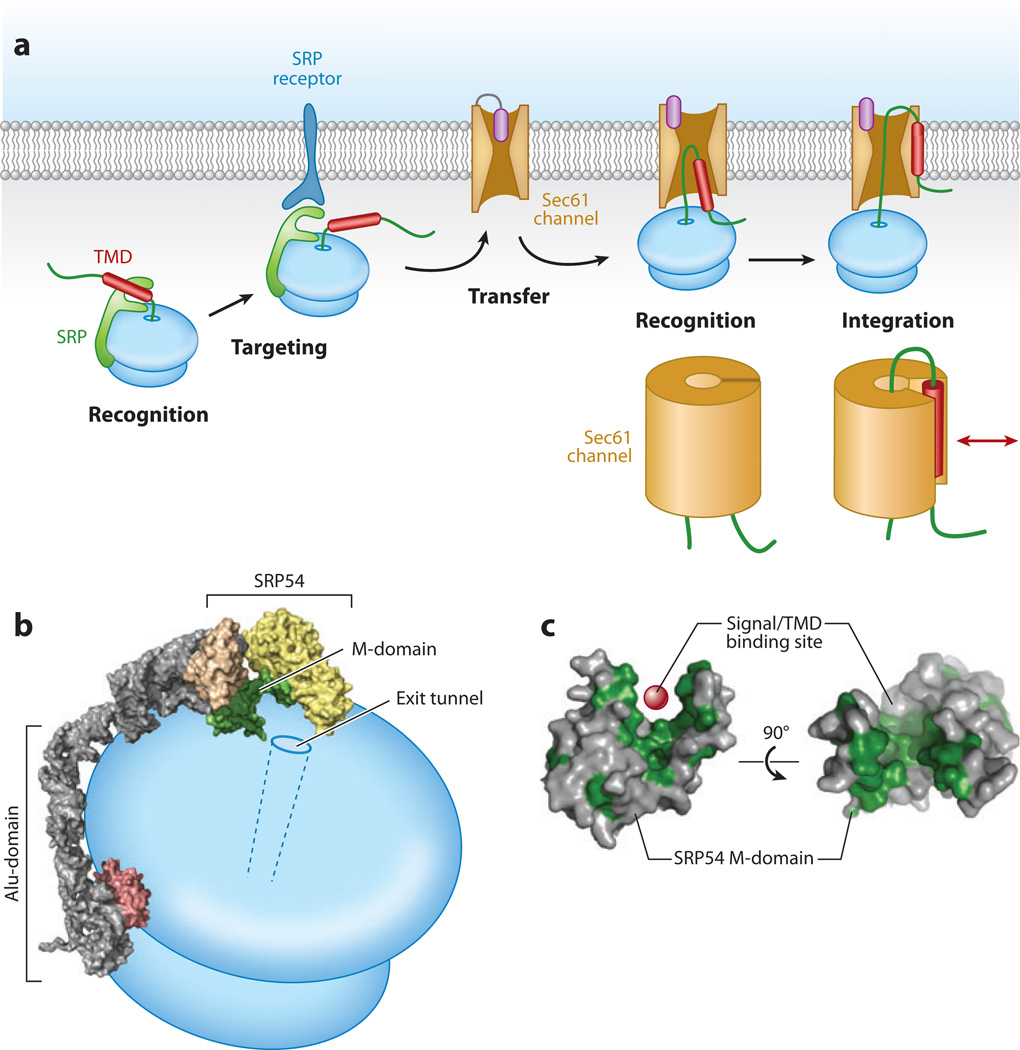
Figure 3.
Basic steps in cotranslational membrane protein insertion. (a) An integral membrane protein (IMP) is recognized via a hydrophobic transmembrane domain (TMD) (or signal sequence) by the signal recognition particle (SRP) on the ribosome. This is targeted to the endoplasmic reticulum (ER) membrane via the SRP receptor (SR) and transferred to the Sec61 translocation channel. The transfer reaction is accompanied by a second recognition step, this time by the Sec61 channel. The TMD is then integrated into the membrane via lateral access to the lipid bilayer provided by the Sec61 channel. Below the last two diagrams are schematic representations of an early and late TMD integration step in which the TMD (red ) is leaving the Sec61 channel via a lateral opening. (b) Schematic diagram of the SRP positioned on the ribosome, taken from cryo-electron microscopy analyses. The signal sequence recognition domain of SRP54 (the M-domain) is indicated in green and is positioned adjacent to the exit tunnel on the ribosome. The portion of the Alu-domain that binds near the elongation factor site to slow translation is shown in pink. (c) The M-domain of SRP54 in two views with hydrophobic residues indicated in green. The red sphere represents the site of signal sequence (or TMD) binding within the hydrophobic groove.