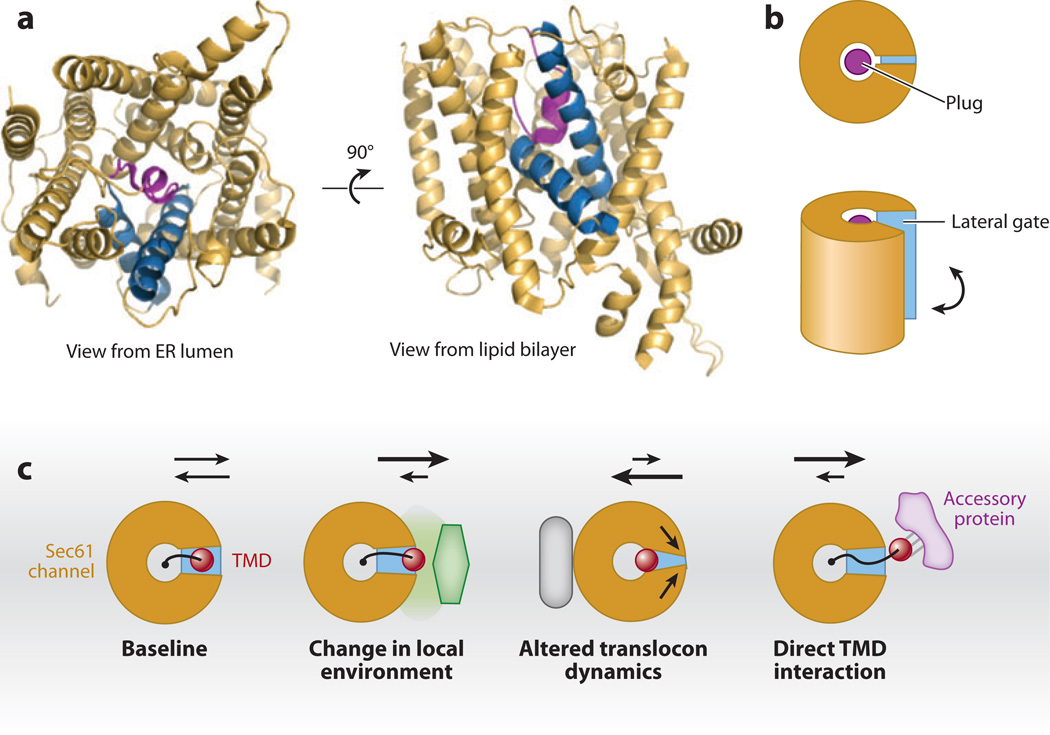
Figure 4.
The Sec61 translocon and potential accessory factors. (a) Structural representation of the archaeal homolog of the Sec61 complex as viewed from the endoplasmic reticulum (ER) lumen and from the plane of the membrane (rotated by 90°). The left and right halves show a pseudo twofold symmetry. The helices are organized around a central pore that is occluded by a short plug helix (purple). The two blue helices are helix 2 and helix 7, which form the so-called lateral gate. When these are separated, they provide a route of access to the lipid bilayer from the central pore. In the lipid bilayer view, note that the plug (which is in the central pore) is directly behind the lateral gate, illustrating how lipid access could be mediated when the gate helices move apart. (b) Schematic representation of the structures in panel a. (c) Schematic representation of how lateral exit of a transmembrane domain (TMD) from the Sec61 channel (as viewed from the ER lumen) could be influenced by trans-acting accessory factors. The baseline model shows the TMD (red ) tethered to a nascent chain (black line). The nascent chain is in the central channel, whereas the TMD is at the lateral gate. The arrows above the diagram indicate the partitioning of the TMD between the lateral gate and surrounding lipid bilayer ( gray plane). This partitioning could be biased toward the membrane if the local environment was altered by either a different lipid composition or a nearby protein. Conversely, a protein that binds to the translocon could influence its dynamic properties, such as opening of the lateral gate, thereby biasing against partitioning into the lipid bilayer. In the last diagram, an accessory protein such as TRAM (translocating-chain associating membrane protein) that directly binds to a TMD could also bias movement of the TMD out of the Sec61 channel.