Abstract
B7-H4 is a member of B7 family of co-inhibitory molecules and B7-H4 protein is found to be overexpressed in many human cancers and which is usually associated with poor survival. In this study, we developed a therapeutic vaccine made from a fusion protein composed of a tetanus toxoid (TT) T-helper cell epitope and human B7-H4IgV domain (TT-rhB7-H4IgV). We investigated the anti-tumor effect of the TT-rhB7-H4IgV vaccine in BALB/c mice and SP2/0 myeloma growth was significantly suppressed in mice. The TT-rhB7-H4IgV vaccine induced high-titer specific antibodies in mice. Further, the antibodies induced by TT-rhB7-H4IgV vaccine were capable of depleting SP2/0 cells through complement-dependent cytotoxicity (CDC) in vitro. On the other hand, the poor cellular immune response was irrelevant to the therapeutic efficacy. These results indicate that the recombinant TT-rhB7-H4IgV vaccine might be a useful candidate of immunotherapy for the treatment of some tumors associated with abnormal expression of B7-H4. [BMB Reports 2014; 47(7): 399-404]
Keywords: B7-H4, Tumor Escape, T-helper Epitope, Tetanus Toxoid, Tumor Vaccine
INTRODUCTION
B7-H4 (also called B7-S1) is a member of B7 family with co-inhibitory function. The human B7-H4 protein consists of 282 amino acids, which contain an N-terminal hydrophobic region, two immunoglobulin (Ig)-like domains (IgV and IgC), and a hydrophobic C terminus. The IgV-like domain of human B7-H4 is primarily responsible for the interaction with receptor and has approximately 90% homology with the same domain in the mouse (1). Studies looking at amino acid homology suggest that human and mouse B7-H4 likely display cross-species receptor binding (2).
Immunohistochemical analysis does not reveal positive staining for B7-H4 protein in all tissues or organs in healthy individuals. But, protein expression can be induced on T cells, B cells, monocytes, and DCs after in vitro stimulation (1, 3). It has been reported that B7-H4 ligation to T cells has a profound inhibitory effect on the cell proliferation, cytokine secretion, and cytotoxicity activity (4, 5), while blocking B7-H4 with specific monoclonal antibodies could promote T cell proliferation and IL-2 production (1). Together, these data suggest that B7-H4 is a negative regulator of T cell immunity. However, the receptor which binds with B7-H4 is still undefined.
Constitutive B7-H4 protein expression can be detected in many cancers such as ovarian, breast and melanoma cancer (4, 6-8). Furthermore, overexpression of B7-H4 protein on cancer cells in some of these malignancies is associated with adverse clinical and pathologic features, including constitutional symptoms, tumor necrosis, and advanced tumor size, stage, and so on (8, 9). Otherwise, downregulation of B7-H4 has been showed to enhance T cell proliferation, decrease apoptosis, stimulate cell cycle progression and elevate cytokine production (10). So, B7-H4 on cancer cells negatively regulates T cell-mediated antitumor immunity.
Besides expressed on tumor cells, B7-H4 was also expressed on the surface of some tumor macrophages (11). Interleukin (IL)-6 and IL-10 in high concentrations in the tumor microenvironment stimulate macrophage B7-H4 expression (11). B7-H4+ tumor macrophages suppressed tumor-associated antigen-specific T cell immunity and blocking B7-H4 restored the T cell stimulating capacity of the macrophages and contributes to tumor regression in vivo (8, 11).
Above all, B7-H4 might be not only regarded as a tumor associated antigen, but also as an immune-inhibitory factor playing a role in immune evasion of tumor cells. Blocking B7-H4 may represent novel strategies to inhibit tumor growth and enhance T cell tumor immunity in cancer at the same time.
Active vaccination against B7-H4 is an approach with many advantages by inducing a polyclonal Ab response, potentially leading to better activation of Ab-dependent effector functions. However, in the case of most tumor Ags, which belong to self Ags, immunological tolerance may hamper vaccination effects because high affinity self reactive Th cells are eliminated. In recent years, the approach of inserting foreign helper epitopes to the self Ags to overcome immunological tolerance has been proven to be very effective for eliciting therapeutic antibodies (12, 13).
In this study, we fused a TT830-843 T-helper cell epitope (QYIKANSKFIGITE), because of its stronger T-helper stimulation and universal activity as a vaccine carrier in human and BALB/c mice (14, 15), to the N terminus of human B7-H4 IgV domain (TT-rhB7-H4IgV), and expressed this protein in Escherichia coli. We next chose the B7-H4 expressing SP2/0 myeloma and its syngeneic host (the BALB/c mouse) as the model to study the anti-tumor activity of the TT-rhB7-H4IgV vaccine. SP2/0 myeloma had been used in the research on B7-H1 vaccine in our lab because it was also a B7-H1-expressing cell line(16). Vaccination with TT-rhB7-H4IgV protein significantly suppressed the growth of SP2/0 myeloma in both preventive and therapeutic mice models and increased survival rate of tumor-bearing mice. Although the cellular immune response appeared negligible in vaccination group mice, the high-titer antibodies might correlate with tumor protection. Our results demonstrated that TT-rhB7-H4IgV protein vaccine has the potential to treat some tumors associated with overexpression of B7-H4.
RESULTS AND DISSCUSION
Vector construction
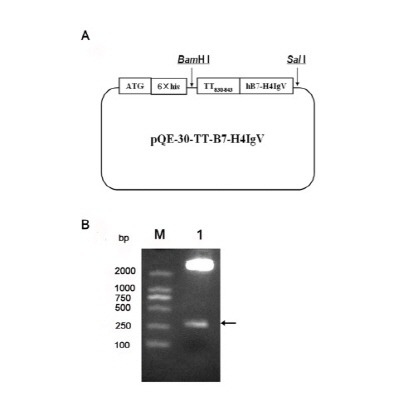
The IgV-like domain of this family plays an important role and is responsible for the interaction of ligands and receptors (17, 18). We expressed the extracellular IgV domain of PD-1 before and this soluble PD-1-IgV protein could bind PD-L1 competitive with surface PD-1 to enhance cytotoxic T lymphocyte (CTL) activity and anti-tumor effect on tumor-bearing mice by inhibiting the PD-L1/PD-1 signal pathway (19). Although the receptor which binds with B7-H4 is still undefined, we choose IgV-like domain again. The DNA fragments encoding TT-rhB7-H4IgV were cloned into a bacterial expression plasmid pQE30 (Fig. 1A) and the recombinant plasmid was confirmed by restriction digestion with BamHI and Sal I (Fig. 1B) and sequenced. B7-H4 belongs to immunoglobulin (Ig) superfamily according to the construction.
Fig. 1. (A) Schematic diagrams of pQE30-TT-rhB7-H4IgV expression vectors. The recombinant genes encoding TT-rhB7-H4IgV were inserted into the pQE-30 vector and expressed in E. coli DH5α under the control of T7 promoter. (B) Restriction analysis of recombinant plasmid pQE30-TT-rhB7-H4IgV. M, DL2000; lane 1, pQE30-TT-rhB7-H4IgV digested with BamHI and SalI. Arrowhead indicates the target gene.
Expression, purification and refolding of TT-rhB7-H4IgV
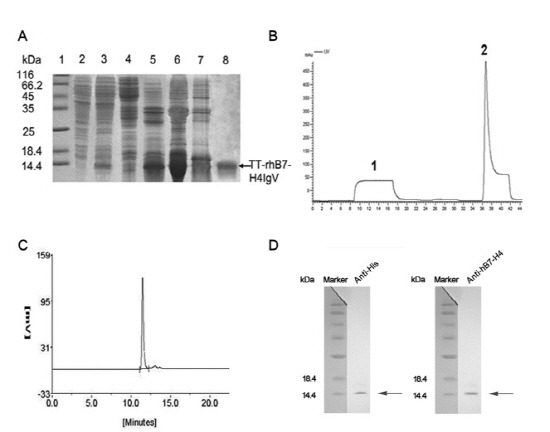
The pQE30-TT-rhB7-H4IgV was transformed into DH5α to express the fusion proteins with an N-terminal six-histidine tag. The expression level was approximately 25% of the total bacteria proteins (Fig. 2A, lane 3) and the observed molecular weight of TT-rhB7-H4IgV was ∼12 kDa, consistent with the expected size. But the proteins formed inclusion bodies in E. coli (Fig. 2A, lane 5) and were purified by Ni2+-chelating affinity chromatography under denaturing conditions (Fig. 2B). Then they were refolded by dialysis. The final yields were 4.5 mg purified protein per gram of cell paste. The purity of the final purified TT-rhB7-H4IgV protein was more than 95% as identified by HPLC (Fig. 2C). The recombinant protein was further analyzed by Western blotting with anti-his antibodies and anti-hB7-H4 antibodies (Fig. 2D).
Fig. 2. Purification and identification of TT-rhB7-H4IgV. (A) SDS-PAGE analysis of TT-rhB7-H4IgV expression in E. coli and purification by nickel (Ni2+) chelate affinity column. Lane 1, molecular weight standards (kDa); lane 2, total cell lysate before induction; lane 3, total cell lysate of pQE30-TT-rhB7-H4IgV after induction; lane 4, supernatant of cell lysate; lane 5, inclusion body after sonication; lane 6, inclusion body after washed with 4 mol/L urea; lane 7,8 contaminated proteins and purified TT-rhB7-H4IgV protein (corresponding to the peak1, 2 in B). (B) Elution profile of the protein by nickel (Ni2+) chelate affinity column. Peak 1, flow through; peak 2, the target protein. (C) SEC-HPLC analysis of the purity of TT-rhB7-H4IgV. 5 μg purified TT-rhB7-H4IgV were analyzed on a G2000SW column, detected at OD280. (D) Proteins were identified by Western blot with anti?his Ab (R94025, Invitrogen, USA) and anti-hB7-H4 Ab (AF1134a, ABGENT, USA). M, Protein molecular weight marker.
Significant growth suppression of SP2/0 myeloma in mice treated with TT-rhB7-H4IgV protein vaccine
We examined the TT-rhB7-H4IgV protein vaccine-induced anti-tumor activity against B7-H4 expressing SP2/0 myeloma established by s.c. inoculation. For the preventive effect of the vaccine, Three groups of 10 BALB/c mice were vaccinated with TT-rhB7-H4IgV protein, rhB7-H4IgV protein (see supplementary result), or only adjuvant respectively. Two weeks later, the mice were challenged with 5 × 106 SP2/0 cells and tumor growth was monitored. All of the mice vaccinated with adjuvant developed large solid tumors within 12-22 days of subcutaneous inoculation. The tumor growth was significantly suppressed in TT-rhB7-H4IgV and rhB7-H4IgV vaccine group. There were 50% (5 of 10) mice and 70% (7 of 10) mice respectively in the two vaccine group developed small, slow growing tumors (Fig. 3A). The tumor in TT-rhB7-H4IgV group grew slower compared with it in rhB7-H4IgV group, although there were no statistically significant. In addition, life span of another three groups of BALB/c mice (n=10) with the same treatment as above was observed. As shown in Fig. 3B, the increase in survival rate in mice vaccinated with TT-rhB7-H4IgV or rhB7-H4IgV vaccine was also statistically significant (P < 0.05), compared with adjuvant group.
Fig. 3. The preventive (A, B) and therapeutic (C, D) effect of TT-rhB7-H4IgV vaccine to transplanted SP2/0 tumor of BALB/c mice. (A, C) Growth of SP2/0 tumors vaccinated with TT-rhB7-H4IgV, rhB7-H4IgV, or only adjuvant. The number of animals that developed tumors/total number of animals is indicated in parenthesis. (B, D) Life span of BALB/c mice after challenge of SP2/0 cells. The survival data were subjected to Kaplan-Meier analysis. *Statistically significant difference compared with the control adjuvant mice (P < 0.05).
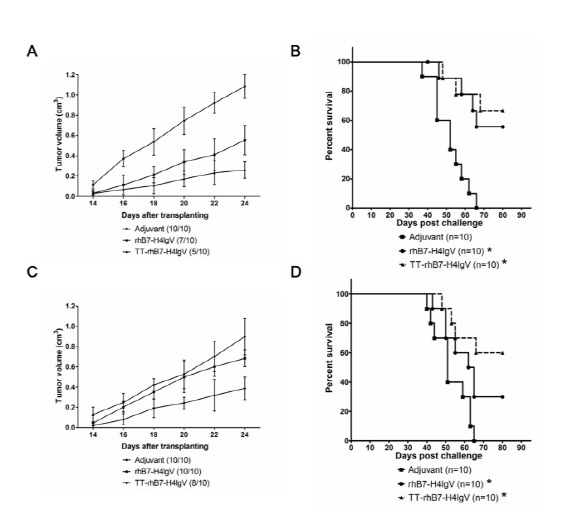
To show the therapeutic effect of the vaccine, we allowed tumors to establish before vaccination. The mice were immunized with TT-rhB7-H4IgV, rhB7-H4IgV protein, or adjuvant respectively until tumor grows to at least 0.5 cm in diameter. As shown in Fig. 3C, only vaccination with TT-rhB7-H4IgV protein had a significantly therapeutic effect on tumor growth. Although the average tumor growth rate in rhB7-H4IgV group was decreased in some instances compared with adjuvant group, there were no statistically significant for the difference. The tumor incidence was reduced only in TT-rhB7-H4IgV group, because 20% (2 of 10) of TT-rhB7-H4IgV vaccinated mice remained tumor free. Furthermore, we observed survival time in another three group of BALB/c mice with the same treatment as above (Fig. 3D) and both TT-rhB7-H4IgV and rhB7-H4IgV vaccination prolonged the life span of tumor bearing mice (P < 0.05).
Humoral and cellular immunity
To investigate the immunological mechanism underlying the therapeutic effect of TT-rhB7-H4IgV vaccine, we measured anti-B7-H4 antibody in mouse serum by ELISA. As shown in Fig. 4A, the TT-rhB7-H4IgV vaccine elicited high-titer anti-hB7-H4 (1 : 100,000) and anti-mB7-H4 (1 : 10,000) antibody responses. We used serum from adjuvant group as another negative control. The anti-hB7-H4 antibody titers obtained from TT-rhB7-H4IgV group were at least 10-fold higher than rhB7-H4IgV group (see supplementary Fig. 2). Combined with the better anti-tumor effect in TT-rhB7-H4IgV group, the data indicated that the TT epitope was efficiently used as vaccine carriers. The results suggested that antibodies induced by TT-rhB7-H4IgV vaccine can interact to human as well as mouse B7-H4 antigens.
Fig. 4. (A) ELISA analysis of antiserum from mice immunized with TT-rhB7-H4IgV. Serum from mice immunized with adjuvant was used as negative control. (B) Determination of binding activity of anti-B7-H4 serum to native B7-H4 on SP2/0 cell surface by FCM. Anti-mB7-H4 mAb was used as positive control and adjuvant serum as negative control. (C) Complement-mediated cytotoxicity. Antiserum elicited by TT-rhB7-H4IgV had similar complement-mediated cytotoxicity of SP2/0 cells to anti-mB7-H4 mAb. Anti-mB7-H4 mAb was used as a positive control and the serum from adjuvant vaccination was used as a negative control. (D) Induction of B7-H4-specific IFN-γ-producing cellular immune responses by TT-rhB7-H4IgV vaccine. The number of IFN-γ-producing B7-H4-specific T cell precursors was determined using ELISPOT assay. TT-rhB7-H4IgV vaccine vs. adjuvant control P > 0.05.
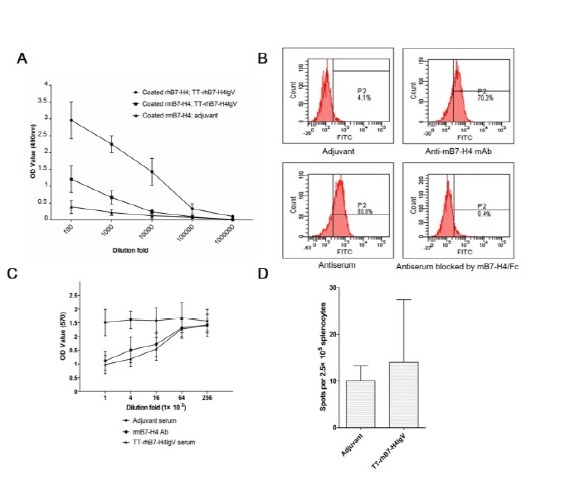
The ability of the serum antibodies to recognize the native cell surface B7-H4 protein was tested by flow cytometry. As shown in Fig. 4B, the serum antibodies could bind to B7-H4-expressing SP2/0 cells (80.8%). To confirm binding specificity, rmB7-H4/Fc protein was used to compete with the native membrane B7-H4. The competitive inhibition rate was 89.6%. The result indicated that the TT-rhB7-H4IgV vaccine could induce antibodies largely to a mixture of native structures.
The mechanisms by which polyclonal anti-B7-H4 Abs induced by vaccination affect tumor growth need to be elucidated. In this study, we examined the effect of B7-H4-expressing SP2/0 cell depletion in vitro through CDC induced by the TT-rhB7-H4IgV antisera. The killing rate of SP2/0 cells reached 48.3 ± 7.5% (1 : 1,000 fold diluted anti-B7-H4 mAb (500 μg/ml) and 53.6±8.4% (1 : 1,000 fold diluted antiserum), respectively (Fig. 4C). The killing rate decreased as dilution of anti-mB7-H4 mAb and antiserum. Otherwise, as discussed above, B7-H4 was also expressed on some macrophages in tumor microenvironment participating T cell stimulating anergy and Treg cell induction (20). Therefore, one possible scenario is that the polyclonal anti-B7-H4 Abs blocked B7-H4 on macrophages to partly reverse the condition of immune suppression in tumor microenvironment and expose tumor cells to the host anti-tumor immunity. The mechanisms of antibodies involvement in the destruction of tumor cells in vivo are need to be investigated in the future.
Further, we analyzed the functional ability of T cells to synthesize cytokine IFN-γ using ELISPOT. As shown in Fig. 4D, there was no significant increase in the number of cells secreting IFN-γ after immunized with TT-rhB7-H4IgV protein vaccine. Therefore, in contrast to DNA vaccines which have been described as a method to induce cellular immune responses (21, 22), it was suggested that cellular immunity might not play important role in mediating protective effects of TT-rhB7-H4IgV protein vaccine.
In conclusion, we designed an TT-rhB7-H4IgV protein vaccine and acquired the purified protein by the way of genetic engineering. Importantly, vaccination with TT-rhB7-H4IgV protein significantly suppressed the growth of SP2/0 myeloma in mice and increased survival rate of tumor-bearing mice. Our results suggested that B7-H4 vaccine might be a potential candidate for tumor immunotherapy. Current studies are underway to investigate further the anti-tumor mechanisms of TT-rhB7-H4IgV vaccine and evaluate TT-rhB7-H4IgV vaccine in primate models in an attempt to develop vaccines useful for treating human B7-H4-expressing tumors.
MATERIALS AND METHODS
Construction of TT-rhB7-H4IgV expression vector
According to the gene sequence of human B7-H4IgV in NCBI databank, TT830-843-hB7-H4IgV gene fragment was synthesized by AuGCT Biotechnology Co. Ltd (China). TT830-843 was at the N-terminal of B7-H4 IgV domain. The derived DNA fragments were digested with BamHI and Sal I (TaKaRa, Japan), and then cloned into similarly digested plasmid pQE-30 (Qiagen, USA). The recombinant plasmid was named pQE30-TT-rhB7-H4IgV and identified using DNA sequencing.
Expression and Purification of TT-rhB7-H4IgV
The plasmid pQE30-TT-rhB7-H4IgV was transformed into E.coli, DH5α host. The transformed colony was inoculated in Luria-Bertani (LB) medium and 0.1 mmol/L isopropyl-1-thio-β-galactopyranoside (IPTG) (Sigma, USA) were added to induce the expression. Cells were harvested and washed twice in buffer A (100 mmol/L NaCl, 50 mmol/L Tris-HCl (pH 7.4), 1 mmol/L EDTA). The washed cell pellets were lysed in buffer A by sonication and centrifuged. The insoluble fractions were washed with 1.5% Triton X-100, 2 mol/L urea. The isolated inclusion bodies were resuspended in solubilizing buffer B (8 mol/L urea, 0.1 mol/L NaH2PO4, 0.01 mol/L Tris-HCl). After centrifuging, the supernatant was filtered and then applied to the nickel (Ni2+) chelate affinity column that was pre-equilibrated with buffer B. Non-specifically bound proteins were removed by extensive wash with 25 mmol/L imidazole in buffer B. Bound proteins were then eluted with 250 mmol/L imidazole in buffer B. The resultant protein was refolded by dialyzing against buffers (0.1mol/L NaCl, and 20 mmol/L Tris-HCl pH 8.0) with decreasing denaturant (6, 4, 2, 0 mol/L urea), and finally against double distilled H2O. After each step, protein samples were centrifuged and supernatants were subjected to the next round of dialysis. The purification of refolded protein was determined by SDS-PAGE.
SEC-HPLC analysis
Size exclusion chromatography high performance liquid chromatography (SEC-HPLC) analysis was performed on a Beckman’s HPLC system (USA). The sample in PBS was injected onto the G2000SW column (TOSOH). Peaks were detected by monitoring at a wavelength of 280 nm. The purity of TT-rhB7-H4IgV was calculated as a percentage of the total peak area detected.
Animal and cell line
Healthy female BALB/c mice (6-8 weeks old; purchased from the National Rodent Laboratory Animal Resource, Shanghai, China) were cared for under institutional animal care protocols in Experimental Animal Center of the Fourth Military Medical University.
SP2/0 (mouse myeloma cell line) was propagated in RPMI 1640 medium (Sigma, USA). The tumor cells were obtained from biotechnology center of the Fourth Military Medical University.
Immunizations
Proteins for immunization were emulsified 1 : 1 (v/v) in IFA (Sigma, USA). Mice were injected s.c. in the neck region with 40 μg of protein in a total volume of 100 μl. Unless otherwise stated, vaccinations were done every other week for a total of three times.
Enzyme-linked immunosorbent assay (ELISA)
The 96-well plates (Costar, USA) were coated with 100 ng rhB7-H4/Fc or rmB7-H4/Fc protein (R&D, USA). Diluted serum samples were added to duplicate wells and were used as primary antibodies. Goat-anti mouse IgG antibody conjugated to HRP (Zhongshan, China) was added as secondary antibody. After washing, 0-phenylenediamine (OPD) substrate buffer was added, and the plates were read at 490 nm in an ELISA plate reader. The experiments were repeated at least three times.
Flow cytometry
SP2/0 were stained with anti-mB7-H4 mAb (Clone 297219, R&D, USA), the antiserum from vaccination or control group followed by incubation with goat antimouse-FITC antibody (Zhongshan, China). The cells were then analyzed on a Becton Dickinson FACS caliber using Cellquest software (Becton Dickinson, San Jose, CA). For blocking the binding capacity of mouse serum, tumor cells were incubated with the antiserum samples which were pretreated with mB7-H4/Fc (R&D, USA) for 30 minutes.
CDC assay
Serum for complement lysis was prepared as follows: blood from three healthy guinea pigs was allowed to clot overnight (4℃) and then centrifuged. SP2/0 cells were routinely cultivated (50 μl of 1 × 106 cells/ml) in round-bottom 96-well plates (Costar, USA). Antiserum dilution (25 μl/well) was added and plates were incubated, followed by the addition of guinea pig serum (50 μl /well, diluted serially) and a second incubation (90min, 37℃, 5% CO2). To determine the CDC of the anti-serum, a MTT test was used to assay cell activity. Anti-mB7-H4mAb (0.1 μg/well) (Clone 297219, R&D, USA) was used as a positive control. The experiments were repeated at least three times.
ELISPOT assay
IFN-γ ELISpot kit (Invitrogen, USA) was used. Splenocytes from vaccinated mice were isolated and restimulated with SP2/0 cells (splenocytes : tumor cells, 30 : 1) and cultured for 24 h. SP2/0 cells were killed by 60 Gy irradiation before and immediately used as antigen presenting cell specifically for B7-H4. The restimulated splenocytes were then added to anti-mouse IFN-γ mAb precoated 96-well plates (2.5 × 105/well) and further incubated at 37℃ for 4 h. The spots were counted using a dissecting microscope. The spot numbers were the mean of triplicates in each vaccinated group.
Statistical analysis
Data were analyzed by Student’s t test. Results were expressed as the means ± standard deviations (SD). Differences were considered significant when the P value was less than 0.05.
Acknowledgments
This study was funded by National Natural Foundation of China, NSFC30900537 and NSFC81001182.
References
- 1.Prasad D. V., Richards S., Mai X. M., Dong C. B7S1, a novel B7 family member that negatively regulates T cell activation. Immunity. (2003);18:863–873. doi: 10.1016/S1074-7613(03)00147-X. [DOI] [PubMed] [Google Scholar]
- 2.Choi I. H., Zhu G., Sica G. L., Strome S. E., Cheville J. C., Lau J. S., Zhu Y., Flies D. B., Tamada K., Chen L. Genomic organization and expression analysis of B7-H4, an immune inhibitory molecule of the B7 family. J. Immunol. (2003);171:4650–4654. doi: 10.4049/jimmunol.171.9.4650. [DOI] [PubMed] [Google Scholar]
- 3.Sica G. L., Choi I. H., Zhu G., Tamada K., Wang S. D., Tamura H., Chapoval A. I., Flies D. B., Bajorath J., Chen L. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. (2003);18:849–861. doi: 10.1016/S1074-7613(03)00152-3. [DOI] [PubMed] [Google Scholar]
- 4.Salceda S., Tang T., Kmet M., Munteanu A., Ghosh M., Macina R., Liu W., Pilkington G., Papkoff J. The immunomodulatory protein B7-H4 is overexpressed in breast and ovarian cancers and promotes epithelial cell transformation. Exp. Cell Res. (2005);306:128–141. doi: 10.1016/j.yexcr.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 5.Krambeck A. E., Thompson R. H., Dong H., Lohse C. M., Park E. S., Kuntz S. M., Leibovich B. C., Blute M. L., Cheville J. C., Kwon E. D. B7-H4 expression in renal cell carcinoma and tumor vasculature: associations with cancer progression and survival. Proc. Natl. Acad. Sci. U.S.A. (2006);103:10391–10369. doi: 10.1073/pnas.0600937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fauci J. M., Straughn J. M., Jr., Ferrone S., Buchsbaum D. J. A review of B7-H3 and B7-H4 immune molecules and their role in ovarian cancer. Gynecol. Oncol. (2012);127:420–425. doi: 10.1016/j.ygyno.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 7.Simon I., Katsaros D., Rigault de la Longrais I., Massobrio M., Scorilas A., Kim N. W., Sarno M. J., Wolfert R. L., Diamandis E. P. B7-H4 is over-expressed in early-stage ovarian cancer and is independent of CA125 expression. Gynecol. Oncol. (2007);106:334–341. doi: 10.1016/j.ygyno.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 8.Quandt D., Fiedler E., Boettcher D., Marsch W., Seliger B. B7-h4 expression in human melanoma: its association with patients' survival and antitumor immune response. Clin. Cancer Res. (2011);17:3100–3111. doi: 10.1158/1078-0432.CCR-10-2268. [DOI] [PubMed] [Google Scholar]
- 9.Chen L. J., Sun J., Wu H. Y., Zhou S. M., Tan Y., Tan M., Shan B. E., Lu B. F., Zhang X. G. B7-H4 expression associates with cancer progression and predicts patient's survival in human esophageal squamous cell carcinoma. Cancer Immunol. Immunother. (2011);60:1047–1055. doi: 10.1007/s00262-011-1017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun S. Q., Jiang C. G., Lin Y., Jin Y. L., Huang P. L. Enhanced T cell immunity by B7-H4 downregulation in nonsmall-cell lung cancer cell lines. J. Int. Med. Res. (2012);40:497–506. doi: 10.1177/147323001204000211. [DOI] [PubMed] [Google Scholar]
- 11.Kryczek I., Zou L., Rodriguez P., Zhu G., Wei S., Mottram P., Brumlik M., Cheng P., Curiel T., Myers L., Lackner A., Alvarez X., Ochoa A., Chen L., Zou W. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J. Exp. Med. (2006);203:871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalum I., Butler D. M., Jensen M. R., Hindersson P., Steinaa L., Waterston A. M., Grell S. N., Feldmann M., Elsner H. I., Mouritsen S. Therapeutic antibodies elicited by immunization against TNF-alpha. Nat. Biotechnol. (1999);17:666–669. doi: 10.1038/10878. [DOI] [PubMed] [Google Scholar]
- 13.Dalum I., Jensen M. R., Gregorius K., Thomasen C. M., Elsner H. I., Mouritsen S. Induction of cross-reactive antibodies against a self protein by immunization with a modified self protein containing a foreign T helper epitope. Mol. Immunol. (1997);34:1113–1120. doi: 10.1016/S0161-5890(97)00147-8. [DOI] [PubMed] [Google Scholar]
- 14.Valmori D., Pessi A., Bianchi E., Corradin G. Use of human universally antigenic tetanus toxin T cell epitopes as carriers for human vaccination. J. Immunol. (1992);149:717–721. [PubMed] [Google Scholar]
- 15.Yu Z., Healy F., Valmori D., Escobar P., Corradin G., Mach J. P. Peptide-antibody conjugates for tumour therapy: a MHC-class-II-restricted tetanus toxin peptide coupled to an anti-Ig light chain antibody can induce cytotoxic lysis of a human B-cell lymphoma by specific CD4 T cells. Int. J. Cancer. (1994);56:244–248. doi: 10.1002/ijc.2910560217. [DOI] [PubMed] [Google Scholar]
- 16.Zhang C., Wang W., Qin X., Xu Y., Huang T., Hao Q., Li W., Wu S., Zhang Y. B7-H1 protein vaccine induces protective and therapeutic antitumor responses in SP2/0 myeloma-bearing mice. Oncol. Rep. (2013);30:2442–2448. doi: 10.3892/or.2013.2686. [DOI] [PubMed] [Google Scholar]
- 17.Suh W. K., Wang S., Duncan G. S., Miyazaki Y., Cates E., Walker T., Gajewska B. U., Deenick E., Dawicki W., Okada H., Wakeham A., Itie A., Watts T. H., Ohashi P. S., Jordana M., Yoshida H., Mak T. W. Generation and characterization of B7-H4/B7S1/B7x-deficient mice. Mol. Cell Biol. (2006);26:6403–6411. doi: 10.1128/MCB.00755-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansen J. D., Du Pasquier L., Lefranc M. P., Lopez V., Benmansour A., Boudinot P. The B7 family of immunoregulatory receptors: a comparative and evolutionary perspective. Mol. Immunol. (2009);46:457–472. doi: 10.1016/j.molimm.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Zhang C., Wu S., Xue X., Li M., Qin X., Li W., Han W., Zhang Y. Anti-tumor immunotherapy by blockade of the PD-1/PD-L1 pathway with recombinant human PD-1-IgV. Cytotherapy. (2008);10:711–719. doi: 10.1080/14653240802320237. [DOI] [PubMed] [Google Scholar]
- 20.Kryczek I., Wei S., Zhu G., Myers L., Mottram P., Cheng P., Chen L., Coukos G., Zou W. Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Res. (2007);67:8900–8905. doi: 10.1158/0008-5472.CAN-07-1866. [DOI] [PubMed] [Google Scholar]
- 21.Lin C. C., Chou C. W., Shiau A. L., Tu C. F., Ko T. M., Chen Y. L., Yang B. C., Tao M. H., Lai M. D. Therapeutic HER2/Neu DNA vaccine inhibits mouse tumor naturally overexpressing endogenous neu. Mol. Ther. (2004);10:290–301. doi: 10.1016/j.ymthe.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 22.Renard V., Sonderbye L., Ebbehoj K., Rasmussen P. B., Gregorius K., Gottschalk T., Mouritsen S., Gautam A., Leach D. R. HER-2 DNA and protein vaccines containing potent Th cell epitopes induce distinct protective and therapeutic antitumor responses in HER-2 transgenic mice. J. Immunol. (2003);171:1588–1595. doi: 10.4049/jimmunol.171.3.1588. [DOI] [PubMed] [Google Scholar]


