Summary
Latent reservoirs of HIV-1 infected cells are refractory to antiretroviral therapies (ART) and remain the major barrier to curing HIV-1. Because latently infected cells are long-lived, immunologically invisible, and may undergo homeostatic proliferation, a “shock and kill” approach has been proposed to eradicate this reservoir by combining ART with inducers of viral transcription. However, all attempts to alter the HIV-1 reservoir in vivo have failed to date. Using humanized mice, we show that broadly neutralizing antibodies (bNAbs) can interfere with establishment of a silent reservoir by Fc-FcR mediated mechanisms. In established infection, bNAbs or bNAbs plus single inducers are ineffective in preventing viral rebound. However, bNAbs plus a combination of inducers that act by independent mechanisms synergize to decrease the reservoir as measured by viral rebound. Thus, combinations of inducers and bNAbs constitute a therapeutic strategy that impacts the establishment and maintenance of the HIV-1 reservoir in humanized mice.
Introduction
HIV-1 infection can be suppressed with combination anti-retroviral therapy (ART). However, therapy must be maintained for the life of the individual because even years of ART does not eliminate a reservoir of latently infected cells harboring replication competent provirus(Siliciano et al., 2003). As a result, ART termination produces rapid viral rebound (Davey et al., 1999). One strategy proposed to eliminate latent viruses involves reversing their latent state using agents that induce HIV-1 RNA synthesis under the cover of ART (Deeks, 2012). However, all attempts to alter the reservoir by intensifying ART with additional anti-retroviral drugs(Dinoso et al., 2009; Gandhi et al., 2010), or administering viral inducers in the presence of ART, have failed to date(Archin et al., 2014; Dybul et al., 2002; Lafeuillade et al., 2001; Prins et al., 1999).
Like ART, broadly neutralizing antibodies (bNAbs) against HIV-1 can completely suppress viremia in HIV-1 infected humanized mice(Horwitz et al., 2013; Klein et al., 2012b) and SHIV infected macaques(Barouch et al., 2013; Shingai et al., 2013). Although the composition of the reservoir is ill defined, and may differ between ART and antibody treatments, discontinuation of ART or bNAb therapy in hu-mice and macaques results in viral rebound, indicating persistence of a functionally silent pool of cells harboring replication-competent virus. Moreover, the relative frequency of latently infected CD4+ T cells as measured by ex vivo re-activation is similar in ART suppressed hu-mice and humans(Chun et al., 1997; Denton et al., 2012; Finzi et al., 1997; Marsden et al., 2012; Wong et al., 1997). Thus, antibodies and ART control HIV-1 infection in humice but allow persistence of a latent reservoir.
Unlike ART however, antibodies can engage the host immune system by virtue of their Fc effector domains(Nimmerjahn and Ravetch, 2008) and thereby accelerate clearance of cell free virus(Igarashi et al., 1999), induce antibody dependent cytotoxicity to kill infected cells(Bonsignori et al., 2012; Chung et al., 2011; Forthal et al., 2013; Forthal et al., 2001; Jost and Altfeld, 2013; Sun et al., 2011), and produce immune complexes that activate dendritic cells to become potent antigen presenting cells(Dhodapkar et al., 2005). Finally, some classes of bNAbs can prevent cell-cell transmission of HIV-1(Abela et al., 2012; Malbec et al., 2013), whereas ART’s activity in this regard is still debated(Agosto et al., 2014; Schiffner et al., 2013; Sigal et al., 2011).
Here we examine the effects of bNAbs on the establishment of the reservoir and on its maintenance in the presence of inducers of viral transcription by measuring viral rebound. We find that bNAbs can interfere with the establishment of the reservoir by a mechanism that depends on their ability to bind to Fc receptors and that bNAbs plus a combination of inducers can reduce viral rebound from the reservoir in established infections in humanized mice.
Results
Post Exposure Prophylaxis with bNAbs
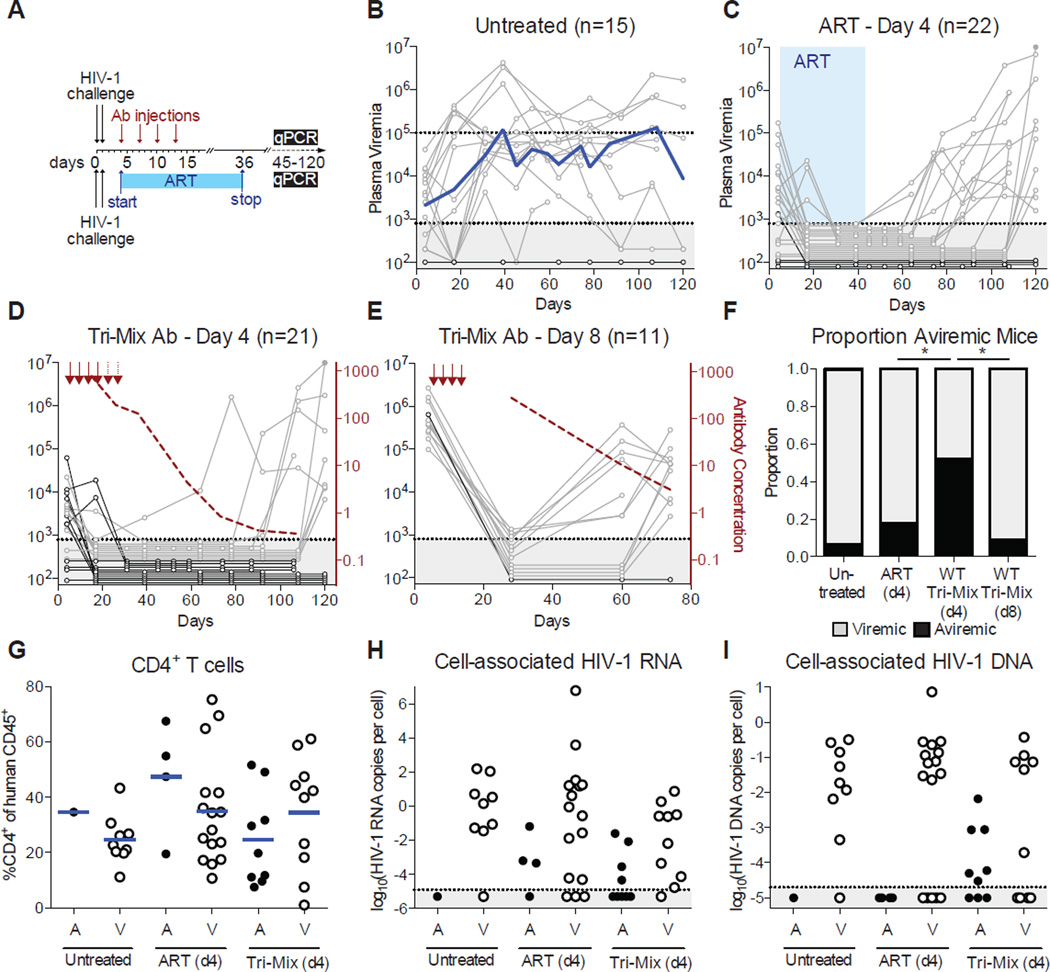
The ART-resistant reservoir is established early in infection as evidenced by post-exposure prophylaxis experiments in humans and macaques(Landovitz and Curry, 2009; Lifson et al., 2000; Tsai et al., 1998; Tsai et al., 1995; Whitney et al., 2014). Post-exposure prophylaxis with ART or previous-generation bNAbs is only effective when administered within 24 hours of intravenous exposure(Ferrantelli et al., 2007; Landovitz and Curry, 2009; Lifson et al., 2000; Nishimura et al., 2003; Tsai et al., 1998; Tsai et al., 1995). To determine if the current generation of more potent bNAbs can abort the establishment of a latent HIV-1 reservoir at later time points, we performed post-exposure prophylaxis experiments in humanized mice (Figure 1A). Mice were infected with HIV-1YU2 (150ng p24) by intraperitoneal injection, and treated with either ART (raltegravir, emtricitabine, tenofovir)(Denton et al., 2012; Nischang et al., 2012) or a tri-mix of bNAbs (3BNC117, 10–1074, and PG16)(Horwitz et al., 2013) 4 or 8 days after infection when viremia was already detectable in 51 of 70 mice. Plasma viremia varied from undetectable to 2.70×106 viral RNA copies/ml at 4 days after infection (Figures 1B–E and Data S1). In the absence of therapy, 14 out of 15 mice in the control group developed sustained plasma viremia ranging from 2.48×103 to 4.19×106 copies/ml (Figure 1B).
Figure 1. Post-exposure prophylaxis with bNAbs.
(A) Schematic timeline for the bNAb (top) and ART (bottom) experiments. (B) Plasma viremia for untreated mice. The x-axis is in days post HIV-1 challenge. The y-axis is viral RNA copies/ml. Gray shading indicates values beneath the detection limit of 800 copies/ml. The blue line indicates the geometric mean plasma viremia. (C) Plasma viremia for ART-treated mice. Graph as in (B). The blue shading indicates the treatment period with ART. (D) Plasma viremia for antibody-treated mice. The red arrows indicate antibody tri-mix injections. The dashed red line shows average plasma antibody concentration (µg/ml) for all mice in the group. (E) Graph as in (D), for mice treated with antibody starting 8 days after HIV-1 challenge. (F) The proportion of mice that were viremic at the terminal point for each treatment group (*, p < 0.05; Fisher’s Exact test) (G) Percentage of CD4+ T cells in the spleen at the terminal point measured by flow cytometry, organized by treatment group. (A = aviremic, V = viremic) (H) Cell-associated HIV-1 RNA measured in spleen T cells at the terminal point, plotted as the ratio of HIV-1 RNA to CCR5 copies for each mouse. Gray shading indicates the detection limit of 1.25×10−5 copies per cell. (I) Cell-associated HIV-1 DNA measured in spleen T cells at the terminal point, plotted as the ratio of HIV-1 RNA to CCR5 copies for each mouse. Gray shading indicates the detection limit of 2.0×10−5 copies per cell. Mice that died prematurely are not included in Figure 1G–I. See also Figures S1, S2 and Data S1.
Doses of ART and antibodies were chosen on the basis of their therapeutic efficacy in chronic HIV-1 infection in hu-mice(Denton et al., 2012; Horwitz et al., 2013; Klein et al., 2012b; Nischang et al., 2012). ART was administered in the food for up to 40 days starting 4 days after infection(Denton et al., 2012; Nischang et al., 2012). Antibodies were administered subcutaneously with a loading dose of 3 mg per mouse, and 3–5 subsequent doses of 1.5 mg each, spaced 3–4 days apart (Figure 1A). Consistent with human and macaque studies, 18 of 22 mice treated with ART showed viremia after ART termination, demonstrating that this form of therapy is relatively ineffective at preventing reservoir development in hu-mice when administered 4 days after infection (Figure 1C). Among the 18 viremic mice, viremia was first detected 28 to 84 days after ART termination (Figures 1C and S1). In contrast, 10 of 21 humice treated with antibodies 4 days after infection showed viremia by the terminal point (p = 0.027, Figure 1F), and for 9 of these 10 viremic mice, the first detectable viremia occurred 74–107 days after the last antibody injection (Figures 1D and S1 and Data S1A). The delay in viral rebound observed for mice treated with antibody at day 4 was statistically significant compared to ART-treated mice (Figure S1). The mice that rebounded showed a geometric mean antibody concentration at rebound of 0.46 µg/ml. However, sustained inhibitory antibody levels did not account for the 11 mice that did not rebound, all of which had antibody levels ≤0.50 µg/ml by termination (Data S1C). In contrast to bNAb treatment at day 4, bNAb treatment after 8 days was far less effective, resulting in viremia in 10 of the 11 treated mice 44–58 days after the last antibody injection (Figures 1E–F and Data S1B).
Mice in the early treatment group that failed to show detectable plasma viremia were further examined for the presence of human CD4+ T cells and cell-associated HIV-1 RNA and DNA in the spleen. We found that mice that failed to develop sustained plasma viremia had similar percentages of CD4+ T cells relative to infected controls, which correlated with absolute CD4+ T cell levels (Figures 1G and S2). Therefore, differences in CD4+ T cell levels are unlikely to account for the observed differences between viremic and aviremic mice (Figure 1G). Moreover, T cell-associated HIV-1 RNA levels were consistent with plasma viral loads, with mice that remained aviremic having either undetectable or lower cell-associated HIV-1 RNA than mice that developed sustained viremia (Figure 1H).
We measured cell-associated viral DNA as an imperfect surrogate of the HIV-1 reservoir. HIV-1 DNA is thought to overestimate the reservoir because it fails to exclude damaged or incomplete viral sequences that cannot be reactivated(Ho et al., 2013). In addition, the overall number cells assayed in mice is limited and therefore the assay is not very sensitive. Nevertheless we found HIV-1 DNA measurements to be consistent with each mouse’s rebound status (Figure 1I). We conclude that bNAbs can interfere with the establishment of the latent HIV-1 reservoir in hu-mice as determined by the significant delay in viral rebound.
Fc Receptor Binding is Required for bNAb Activity
To determine if the efficacy of bNAbs is dependent on the antibodies’ ability to engage components of the immune system through their Fc domains, we repeated the day 4 post-exposure prophylaxis experiments using the same tri-mix of bNAbs carrying Fc region mutations that abrogate both human and mouse Fc-receptor binding (G236R/L328R; GRLR, herein referred to as FcRnull)(Horton et al., 2010). Despite equivalent neutralizing activity in TZM-bl assays(Pietzsch et al., 2012), FcRnull antibodies were far less potent than controls in vivo (Figure 2 and Data S2). Mice treated with FcRnull tri-mix initially suppressed viremia at the same rate as the wild type antibody-treated mice (Figure 2A). However 9 of 15 mice receiving post exposure prophylaxis with the FcRnull tri-mix showed viral rebound by 44 days after the last antibody injection. In contrast, 44 days after the last injection of control antibodies, only 1 of 21 mice showed rebound viremia (p = 0.0004). Not only was the delay in viral rebound significantly reduced for FcRnull antibodies, but the antibody levels at the time of viral rebound were ~50-fold higher for mice receiving FcRnull tri-mix compared to wild-type tri-mix (p = 0.0007, Figure 2B). This suggests FcRnull antibodies have reduced activity in vivo, and thus Fc function enhances antibody activity but is not an absolute requirement.
Fig. 2. FcRnull antibodies suppress viremia but do not prevent rebound.
(A) Plasma viremia as in Fig. 1D for mice treated with FcRnull tri-mix. (B) For all viremic mice, plasma antibody concentration (µg/ml) on the day of viral rebound. Antibody levels were significantly higher in FcRnull tri-mix treated mice compared to wild-type tri-mix treated mice (*, p < 0.05; ***, p < 0.001; Mann-Whitney test). (C) Sequences of gp120 cloned from plasma. Horizontal lines denote individual clones, grouped by mouse, shown on the right. Red ticks and green ticks indicate non-synonymous and synonymous substitutions relative to gp120YU2, respectively. Blue shading highlights sites of mutations that confer escape to the antibody tri-mix. See also Data S2.
The escape variants to the individual bNAbs in the tri-mix used in these experiments have been documented extensively(Horwitz et al., 2013; Klein et al., 2012b). However, we have never observed HIV-1 escape by mutation to the bNAb tri-mix. Rather viral rebound is usually due to a drop in antibody concentrations to sub-therapeutic levels(Horwitz et al., 2013; Klein et al., 2012b). Because mice receiving FcRnull tri-mix showed viral rebound in the presence of antibody concentrations far higher than the therapeutic threshold for wild-type antibodies (Figure 2B), we cloned and sequenced gp120 from the 9 mice that rebounded by day 44 to examine the mechanism for viral breakthrough in the presence of FcRnull tri-mix (Figure 2C). Among all 40 clones sequenced, not a single clone had the triple combination of signature mutations that confer escape to the antibody-tri-mix. We conclude that viral rebound in FcRnull tri-mix treated mice is not attributable to antibody escape, but rather reduced antibody potency. Thus, FcRnull mutant antibodies, which cannot engage Fc receptors, are less active in suppressing infection than their wild type counterparts, and optimal post-exposure prophylaxis by bNAbs requires engagement of Fc-receptors.
Combination Therapy with bNAbs and Inducers
A small number (~15%) of chronically infected hu-mice and macaques treated with antibodies fail to show rebound viremia after therapy is discontinued(Barouch et al., 2013; Horwitz et al., 2013; Klein et al., 2012a; Shingai et al., 2013). This suggests that antibodies may be able to decrease the size of the reservoir, or interfere with its maintenance, in established infections. To determine whether agents that induce viral transcription from latently infected cells can enhance this effect we combined antibody therapy with viral inducers (Figure 3 and Data S3).
Figure 3. Rebound viremia after therapy with single inducers.
(A) Schematic timeline of the experiment. (B–D) Graphs show plasma viremia for individual mice on the left y-axis, geometric mean antibody level (µg/ml) on the right y-axis among all mice in the group (red). The x-axis represents days relative to the first antibody injection. Antibody injections are indicated with red arrows. Mice that had rebound plasma viremia are shown in gray. Mice that failed to rebound are shown in black. (B) Mice that received tri-mix antibodies, but no inducers. (C) Mice that received tri-mix antibodies and vorinostat (green arrows). (D) Mice that received tri-mix antibodies and I-BET151 (purple shading). (E) Mice that received tri-mix antibodies and α (orange arrows). See also Data S3, Figures S3 and S5.
Hu-mice with established HIV-1YU2 infections (viremia ranging from 4.70×103–7.96×105 copies/ml at 2–3 weeks after infection, Figures 3B–E) were treated with tri-mix bNAbs. When plasma viremia and intracellular HIV RNA dropped below detection, they were co-administered a viral inducer for 5–14 days, and monitored for viral rebound for an additional 47–85 days (Figures 3B–E and Figure S3). The inducers tested were vorinostat, an HDAC inhibitor(Archin et al., 2009a; Archin et al., 2009b; Contreras et al., 2009), I-BET151, a BET protein inhibitor(Boehm et al., 2013b; Dawson et al., 2011), and α, a T-cell inhibitory pathway blocker(Alegre et al., 2001; Krummel and Allison, 1995). They were selected because of their documented abilities to induce HIV-1 transcription in vitro, as well as their safety and established pharmacokinetic properties in mice(Krejsgaard et al., 2010; Kwon et al., 1997; Nicodeme et al., 2010).
Hu-mice receiving antibodies plus vorinostat showed no significant differences in viral rebound compared to hu-mice receiving antibody alone (Figures 3B–C and Data S3). The same result was seen for hu-mice treated with antibodies plus I-BET151 or α (Figures 3D–E and Data S3). All 10 mice that received antibody therapy plus vorinostat showed viral rebound when the antibody dropped below therapeutic levels. Of 12 mice that received antibody therapy plus I-BET151, 11 had viral rebound, and 10 of 11 mice that received antibody plus α showed viral rebound. In total, of 33 mice that received antibody plus a single inducer, 31 showed viral rebound. In comparison, of 25 mice that received antibody therapy alone, 22 rebounded after the level of passively administered antibody decayed below the therapeutic threshold (p = 0.64, Fisher’s Exact Test).
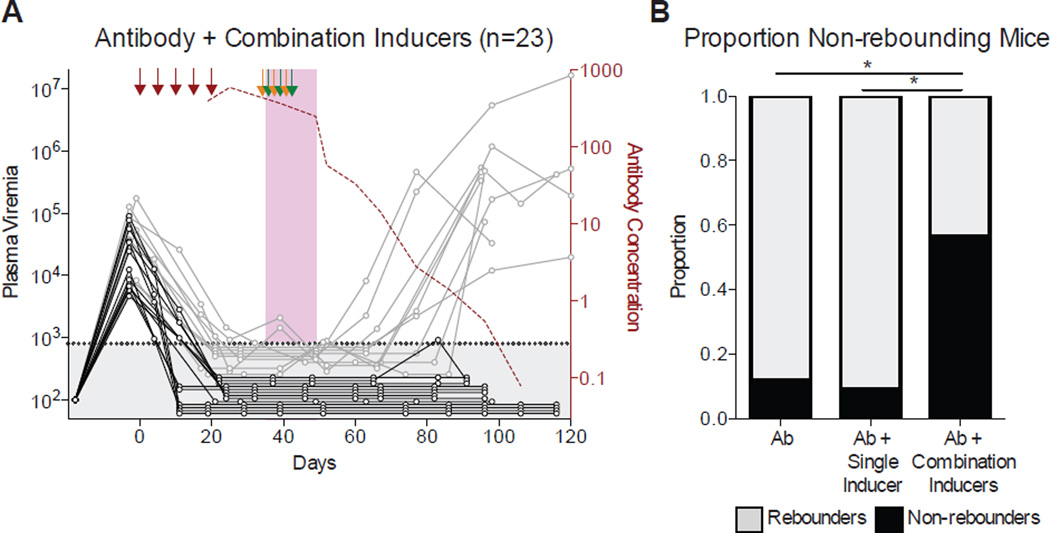
To determine whether a combination of inducers might be more effective than a single inducer, we administered all three inducers simultaneously (Figures 4 and Data S4). In the absence of antibody therapy, the combination of all three inducers did not alter the human graft and did not abort or noticeably alter active infection (Figure S4). Additionally, the human graft did not differ between groups of mice that received antibody alone versus antibody plus the combination of inducers (Figure S4). 23 mice that initially suppressed viremia on antibody therapy were treated with the inducers combination and followed for 62–105 days after the last antibody injection (Figure 4A). Only 10 of the 23 mice (43%) showed viral rebound, while the remaining 57% of mice failed to rebound, a significant decrease in rebound frequency compared to antibody alone (p = 0.0018, Fisher’s Exact Test), or antibody plus a single inducer (p = 0.0001, Fisher’s Exact Test) (Figures 4B and S5).
Figure 4. Combination inducers decrease the incidence of rebound viremia.
(A) Mice treated with tri-mix of antibodies, and a combination of three inducers. Graph, arrows, and shading are as in Figure 3. (B) Graph shows the proportion of mice that showed rebound viremia for each treatment group, where all mice that received antibody tri-mix and any one of the three single inducers (shown in Figure 3C–E) are pooled together (*, p < 0.05; Fisher’s Exact test). See also Data S4, Figures S3, S4 and S5.
Importantly, when compared to antibody alone, neither single inducers nor combination inducers measurably altered the frequency of CD4+ T cells remaining at the end of the experiment (Figure 5A). Additionally, spleen T-cell associated viral RNA reflected plasma viral RNA levels at the time the experiment was terminated in that it was largely undetectable in mice that failed to rebound (Figure 5B).
Figure 5. Antibody persistence and premature termination do not account for non-rebounding.
(A) Percentage of CD4+ T cells at the terminal point measured in the spleen by flow cytometry. (B) Cell-associated HIV-1 RNA measured in spleen cells at terminal point, plotted as the ratio of HIV-1 RNA to CCR5 DNA copies for each mouse. Mice that had measureable HIV-1 RNA, but undetectable CCR5 DNA are plotted as 104 copies per cell. (C) Plasma viremia before therapy was initiated for each mouse, organized by treatment group and rebound status (N.R. = non-rebounder, Reb. = viral rebounder). There was no significant difference for any individual group (Kruskal-Wallis test). (D) The plasma antibody level (µg/ml) at the time of viral rebound for each mouse that rebounded, organized by treatment group. The mean plasma antibody level at the time of rebound was 2.97 µg/ml for all groups. (E) For each mouse that rebounded, the number of days that elapsed from when the antibody level dropped below 2.97 µg/ml to the time of rebound. (F) For mice that did not rebound, the number of days that elapsed from when each mouse’s antibody levels dropped below 2.97 µg/ml to the terminal point. (F) Cell-associated HIV-1 DNA measured in spleen T cells at the terminal point, plotted as the ratio of HIV-1 DNA to CCR5 copies for each mouse. Mice that had measureable HIV-1 DNA, but undetectable CCR5 DNA are plotted as 104 copies per cell. Mice that died prematurely are not included in Figure 5A–B.
Finally, when compared to controls, hu-mice that failed to rebound after combination antibody and inducer therapy showed similar initial plasma viremias to mice that rebounded across all experimental groups (Figure 5C). Therefore, neither initial viremia levels, nor CD4+ T cell levels can account for the differences between the experimental groups.
To determine if antibody persistence or premature termination accounted for differing viral rebound outcomes, we calculated antibody levels at the time of rebound and at the terminal point for 59 of the 63 rebounding mice. The average plasma antibody concentration at the time of viral rebound was 2.97 µg/ml (Figure 5D). Since the antibody concentrations decayed to 2.97 µg/ml at different rates in individual mice, we calculated the number of days that elapsed from when each individual mouse’s antibody levels reached 2.97 µg/ml to when the mouse showed rebound viremia. 50 of 59 mice rebounded within 10 days (Figure 5E). Of the 18 non-rebounding mice, the average antibody concentration at the terminal point was 0.44 µg/ml, with 15 out of 18 mice having antibody concentrations less than 2.97 µg/ml. Furthermore, in non-rebounding mice, an average of 20.2 days elapsed from the time antibody concentrations reached 2.97 µg/ml to termination (Figure 5F). Thus, failure to rebound cannot be explained by antibody persistence or premature termination.
Finally, we could not detect viral DNA at the terminal point in the majority of mice that did not rebound, whereas the majority of mice that did rebound had detectable HIV-1 DNA, with an average of 0.09 copies per T cell (Figure 5G). We conclude that combining vorinostat, I-BET151 and α with immunotherapy decreases the frequency of viral rebound in hu-mice.
Discussion
Eliminating the HIV-1 reservoir in chronic infection is essential to curing the disease, but direct measurement of the latent reservoir to evaluate therapeutic eradication strategies remains difficult(Siliciano and Siliciano, 2013). Quantitative viral outgrowth assays and PCR-based assays of integrated DNA yield disparate results(Eriksson et al., 2013), in part because PCR cannot distinguish between inactive and permanently disabled proviruses, while outgrowth assays underestimate reservoir size(Ho et al., 2013). Indeed, the best way to determine whether a persistent reservoir exists is to measure viral rebound after discontinuing therapy in vivo.
Since the precise cellular and molecular nature of the HIV-1 reservoir is debated, it is not possible to know with certainty how its composition might differ between humans, macaques and hu-mice. Hu-mice resemble infected humans in that they contain human cells that are infected with authentic HIV-1(Brehm et al., 2014; Hatziioannou and Evans, 2012). In addition, the kinetics of viral rebound in hu-mice after suppression of viremia with ART resembles infected humans(Horwitz et al., 2013; Nischang et al., 2012). However, human hematopoietic reconstitution in mice is incomplete, thus important cellular elements of the reservoir may be absent in hu-mice. Additionally, the human graft and the infection can only be maintained in mice for a limited time, making it impossible to distinguish between integrated and unintegrated forms of HIV-1 latency(Bukrinsky et al., 1991).
The macaque model is valuable because it represents an immunologically intact host that may harbor reservoirs found in humans but not in mice. However, the infection in macaques involves non-human primate cells with SHIV or SIV, which differ significantly from HIV-1 molecularly and in their response to drug therapy. Thus, neither of the two model systems is entirely faithful to the human infection. Nevertheless, the two models have produced very similar results in both immunotherapy and prevention experiments to date(West et al., 2014). Whether our results with inducers will translate to infected humans can only be determined in clinical studies.
Despite the potential differences between available models, our experiments indicate that bNAbs can interfere with the establishment of the reservoir in humanized mice when administered early in the infection. One of the key differences between antibodies and ART is that antibodies can engage a variety of host immune effector pathways by way of their Fc receptors(Nimmerjahn and Ravetch, 2008). Consistent with this important difference, our experiments show that the mechanism by which antibodies suppress active infection is dependent on their ability to engage components of the immune system by binding to Fc receptors. Engagement of these receptors is also implicated in antibody-mediated protection against infection in mice and macaques(Hessell et al., 2007; Pietzsch et al., 2012). However, in contrast to the relatively modest effect of FcR engagement on prophylaxis against initial infection(Hessell et al., 2007; Pietzsch et al., 2012), the ~50-fold effect on the dose of antibody required to suppress viral rebound after early therapy is impressive. One potential explanation for the difference in relative antibody potency for prevention versus suppression of rebound viremia is that the effects on the latter are compounded over a far longer period of antibody therapy(McMichael et al., 2010).
One of the strategies proposed to eliminate latent viruses involves inducing their expression under the cover of ART. In theory, this would kill actively infected cells while preventing the spread of infection(Deeks, 2012). In vitro experiments indicate that silent proviruses can in fact be induced to become active by a variety of different agents that impact viral transcription(Bullen et al., 2014; Ho et al., 2013). However, whether reactivated cells will die by the cytopathic effect in the presence of ART has recently been called into question(Shan et al., 2012). Of the three inducers that we tested, vorinostat is the only one that has been studied in HIV-1 infected humans for this purpose. Infected individuals treated with vorinostat plus ART showed a transient increase in resting CD4+ T cell-associated HIV-1 RNA, but no change in plasma viremia, or the frequency of replication-competent HIV-1 within resting CD4+ T cells(Archin et al., 2014; Archin et al., 2012). Our results in hu-mice are consistent with the human studies, and extend them to additional candidate inducers, demonstrating that administration of a single inducer has no significant effect on the ability of the latent reservoir to produce rebound viremia. Although we could not detect an increase in viremia following administration of either single or combination inducers, antibodies were still present and may have interfered with our ability to detect the virus(Igarashi et al., 1999).
Antibody and inducer combinations have not been optimized and we cannot explain why 43% of mice receiving antibodies plus combination inducers continue to rebound. Nor have we addressed the mechanism that would explain the difference between single and combination inducers. However, experiments using cell lines that contain artificial indicators of HIV-1 latency indicate that HDAC and BET protein inhibitors show synergy with transcriptional activators in re-activating HIV-1 in vitro(Boehm et al., 2013a; Dar et al., 2014; Quivy et al., 2002; Reuse et al., 2009). Consistent with the in vitro experiments, the combination of inducers appears to be synergistic in vivo since single inducers had no measurable effect above the background controls, while ~57% of the humice treated with antibodies plus combination inducers failed to rebound. Irrespective of the mechanism, the reduction in viral rebound suggests that the reservoir of HIV-1 infected cells remaining after combination inducer and antibody therapy in hu-mice is significantly decreased, establishing the principle that the HIV-1 reservoir can be altered by combination therapy with antibodies and inducers in vivo.
Experimental Procedures
Mice
NOD Rag1−/−Il2rgNULL (NOD.Cg-Rag1tm1Mom Il2rgtm1Wjl/SzJ, NRG) mice were purchased from The Jackson Laboratory. All mice were and bred and maintained at the Comparative Bioscience Center of The Rockefeller University according to guidelines established by the Institutional Animal Committee. All experiments were performed with authorization from the Institutional Review Board and the IACUC at The Rockefeller University.
Humanized mice
Humanized mice were generated as previously described(Klein et al., 2012b). Briefly, human fetal livers were obtained from Advanced Bioscience Resources (ABR). Fetal livers were homogenized and incubated in HBSS media with 0.1% collagenase IV (Sigma-Aldrich), 40 mM HEPES, 2 mM CaCl2 and 2 U ml−1 DNAase I (Roche) for 30 minutes at 37° C. Hematopoietic Stem Cells (HSCs) were isolated from digested liver using CD34+ HSC isolation kit (Stem Cell Technologies). Neonatal NRG mice (1–5 days old) were sublethally irradiated with 100 cG and injected intrahepatically with 2×105 human CD34+ HSCs 6 hours after irradiation.
Mouse screening for humanization
Eight or more weeks after HSC injection, mice were screened for the presence of human lymphocytes in peripheral blood by flow cytometry. 200 µl whole blood was collected by facial vein bleed and peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation using Ficoll-Paque Plus (GE Healthcare Life Sciences). PBMCs were stained with antibodies to mouse CD45-PECy7, human CD45-Pacific Orange, human CD3-Pacific Blue, human CD19-APC, human CD4-PE, human CD8-FITC, and human CD16-Alexa700 for 25 min at 4 °C. Cells were washed and fixed using Cytofix/Cytoperm (BD Biosciences). Flow cytometry analysis was performed with a LSRFortessa (BD) and FlowJo software (Tree Star). For each mouse, the percentage of human lymphocytes [(100 × human CD45+) / (human CD45+ + mouse CD45+)], termed huCD45+ %, and the percentage of human CD4+ T cells (100 × human CD45+CD3+CD4+ / human CD45+), termed huCD4+ %, was calculated. Mice with at least 10% huCD45+ and 10% huCD4+ were selected for post-exposure prophylaxis experiments, and infected with two doses of HIV-1YU2 (150 ng p24) by i.p. injections, 24 hours apart. Pre-treatment viremia was measured at 72–96 hours following the first HIV-1YU2 injection, and treatment was initiated 4 days following the first injection. For experiments assessing the effects of bNAbs and inducers on established infections, mice with measurable human CD4+ cells by FACS were injected with two doses of HIV-1YU2 (150 ng p24), and pre-treatment viremia was measured 14–18 days after the first injection. Mice with plasma viral loads >3000 RNA copies/ml were selected to receive antibody therapy. After five subcutaneous antibody injections (see below), post-treatment viremias were measured. Only mice with completely suppressed plasma viremias were selected for further analysis and to receive viral inducers.
Plasma viral load measurements
300–500 µl of whole blood was collected from mice at each time point by facial vein bleed. Whole blood was spun at 300g for 10 minutes to separate plasma from the cellular fraction. Total RNA was extracted from 100 µl plasma using QIAmp MinElute Virus Spin Kit (Qiagen) in combination with RNase-free DNase (Qiagen), eluted in a 50 µl volume. HIV-1 RNA was quantified by qRT-PCR. The reaction mixture was prepared using TaqMan RNA-to-Ct 1-Step kit (Applied Biosystems), with 20 µl of eluted RNA, and a sequence specific probe targeting a conserved region of the HIV-1 pol gene (/HEX/5’-CCCACCAACARGCRGCCTTAACTG-3’/ZenDQ, HXB2 nt 4603 to 4626) (Integrated DNA Technologies). Forward and reverse primer sequences were 5’-TAATGGCAGCAATTTCACCA-3’ (HXB2 nt 4577–4596) and 5’-GAATGCCAAATTCCTGCTTGA-3’ (HXB2 nt 4633 to 4653), respectively. Cycle threshold (Ct) values were calibrated using standard samples with known amounts of absolute viral RNA copies. The quantitation limit was previously determined to be 800 copies/ml(Klein et al., 2012b).
Gp120 Sequencing
Gp120 cloning and sequencing was performed as previously described(Klein et al., 2012a). Briefly, cDNA was synthesized from viral RNA using SuperScript III reverse transcriptase (Invitrogen Life Technologies). cDNA was amplified with Expand Long Template PCR System (Roche) with nested PCR. Primers for the first round of PCR were 5’-GGCTTAGGCATCTCCTATGGCAGGAAGAA-3’ and 5’-GGTGTGTAGTTCTGCCAATCAGGGAAGWAGCCTTGTG-3’. Primers for the second round of PCR were 5’-TAGAAAGAGCAGAAGACAGTGGCAATGA-3’ and 5’-TCATCAATGGTGGTGATGATGATGTTTTTCTCTCTGCACCACTCTTCT-3’. Gel-purified PCR amplicons were ligated into pCR4-TOPO (Invitrogen) and transformed into One Shot TOP10 cells. Individual colonies were sequenced using M13F and M13R primers. Sequences were aligned to gp120YU2 (accession number M93258) and analyzed for mutations using Los Alamos Highlighter tool (http://www.hiv.lanl.gov/content/sequence/HIGHLIGHT/HIGHLIGHT_XYPLOT/highlighter.html).
Cell-associated HIV-1 RNA
The cellular fraction of whole blood was resuspended in 400 µl PBS and PBMCs were isolated by density gradient centrifugation as described above. Lymphocytes were split into two samples, one for cell-associated HIV-1 RNA measurements, and one for cell-associated HIV-1 DNA measurements. Cell-associated RNA was extracted and quantified by the same procedures as described above for plasma viral RNA. The lower limit of detection was determined to be 10 copies viral RNA per qRT-PCR reaction. Cell-associated HIV-1 RNA is reported as the ratio of HIV-1 RNA copies per sample to CCR5 genomic DNA copies per equivalent sample measured in DNA extract. For terminal point measurements, spleen tissue was isolated, homogenized, and filtered through 40 µm mesh. Splenocytes were used to isolate HIV-1 RNA as described above.
Cell-associated HIV-1 DNA
PBMCs were isolated from whole blood as described above. Splenocytes were isolated from spleen as described above. Total DNA was extracted using QIAmp DNA Blood Mini Kit (Qiagen) and eluted in 80 µl volume. Purified DNA was quantified for HIV-1 DNA by qPCR using the primers and probe for HIV-1 RNA quantification mentioned above. Genomic human CCR5 DNA was quantified with primers 5’-GTTGGACCAAGCTATGCAGGT-3’ (forward) and 5’- AGAAGCGTTTGGCAATGTGC-3’ (reverse), and the sequence-specific probe /HEX/5’-TTGGGATGACGCACTGCTGCATCAACCCCA-3’/ZenDQ. All qPCR reactions contained 25 µl AmpliTaq Gold PCR master mix (Applied Biosystems), in 50 µl reaction volume. Reaction mixtures were as previously described(Horwitz et al., 2013). HIV-1 DNA is reported as copies per sample to CCR5 genomic copies per equivalent sample.
Terminal Graft
The presence of human lymphocytes at the terminal point was quantified from the spleen and PBMCs by flow cytometry. Isolation of PBMCs and splenocytes were as described above. Staining procedures were as described above.
Antibody concentrations
Plasma levels of passively administered antibodies were quantified by two independent methods. gp120-specific ELISA was as previously described(Klein et al., 2012b), using 10–1074 and 3BNC117 monoclonal antibodies as standard controls. The detection limit was 0.05 µg/ml. Because PG16 does not bind gp120, and endogenously produced gp120-reactive antibodies could confound the ELISA measurement, plasma antibody levels were also quantified by TZM-bl neutralization using the Tier 2 envelopes 3301.v1.c24 and YU2. A mixture with known amounts of 3BNC117, 10–1074, and PG16 was used as standard for calibration.
Day of viral rebound and antibody level at rebound
Plasma viremias immediately preceding and following viral rebound were plotted on a semi-log-y-axis versus days post initial antibody injection (x-axis) for each individual mouse. The linear portion of viremia was fit to a line by least-squares linear regression. The day that viremia crossed the 800 copies/ml quantitation limit, termed rebound day, was calculated from the viremia fit. The antibody concentrations (as determined by TZM-bl neutralization) spanning before and after viral rebound were plotted on a semi-log-y-axis versus days post initial antibody injection. The linear portion of antibody concentrations was fit to a line by least-squares linear regression, and the antibody concentration on the rebound day was calculated from the fit.
Anti-retroviral therapy
Individual tablets of tenofovir disproxil-fumarate (TDF; Gilead Sciences), emtricitabine (FTC; Gilead Sciences), and raltegravir (RAL; Merck) were crushed into fine powder and manufactured with TestDiet 5B1Q feed (Modified LabDiet 5058 with 0.12% amoxicillin) into ½” irradiated pellets. Final concentrations of ART drugs in the food were 720 mg/kg TFV, 520 mg/kg FTC, and 4800 mg/kg RAL. Doses were chosen based on suppression of viremia in humanized mice as previously published(Denton et al., 2012; Nischang et al., 2012), and by pharmacokinetic analysis of these drugs in humanized mice (unpublished, Speck Laboratory). To test potential toxicity, or reduced preference for drug-supplemented food, mice were weighed daily on normal diet, then switched to ART feed and weighed daily. There were no visible signs of toxicity and mice maintained their weights. Assuming mice weigh 25 grams and eat 4 grams of food per day, the drug doses correspond to 2.88 mg/kg TFV, 83 mg/kg FTC, and 768 mg/kg RAL daily.
Antibody therapy
Plasmids encoding 10–1074 or PG16 heavy- and light- chain Ig genes were transfected into HEK 293E cells. Antibodies were isolated from tissue-culture supernatant using Protein G Sepharose 4 Fast-Flow (GE Healthcare). Antibodies were then buffer-exchanged into PBS and sterile-filtered using Ultrafree-CL centrifugal filters (0.22µm; Millipore). Endotoxin was removed from antibody preparations using Triton X-114 (Sigma-Aldrich) as previously described(Aida and Pabst, 1990), and antibodies were concentrated to 10 mg/ml. Sterile, endotoxin-free 3BNC117 (20 mg/ml) was obtained from CellDex Therapeutics. All antibodies were injected subcutaneously as described.
Inducers
Vorinostat (Selleckchem) was suspended in sterile water or sterile water plus 0.5% methylcellulose, 0.1% Tween (v/v) and administered by oral gavage at doses of 60 mg/kg(Krejsgaard et al., 2010). For each mouse, three total doses were administered, spaced 48 hours apart. 100 µg doses of αCTLA4 were injected intraperitoneally (i.p.). Three total doses were administered, spaced 48 hours apart. I-BET was obtained from GlaxoSmithKline and dissolved in 10% beta-cyclodextrin, 5% DMSO in 0.9% saline and injected daily for 14 days at doses of 30 mg/kg(Dawson et al., 2011).
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 6.0 for Mac OS X.
Supplementary Material
Highlights.
bNAbs can be used for post-exposure prophylaxis (PEP) in humanized mice
bNAb PEP efficacy requires Fc-receptor binding
bNAbs plus a single inducer of HIV-1 transcription do not reduce viral rebound
bNAbs plus a combination of inducers significantly reduce viral rebound
Acknowledgements
We thank Roberto Speck and Myburgh Renier for help manufacturing ART feed; CellDex Therapeutics for providing 3BNC117; and all members of the Nussenzweig Laboratory for helpful discussion and advice. This work was supported by the National Institutes of Health (NIH) Medical Scientist Training Program grant T32GM07739 to the Weill Cornell/Rockefeller/Sloan-Kettering Tri-Institutional MD-PhD Program (A.H.-S.); Bill and Melinda Gates Foundation with Comprehensive Antibody Vaccine Immune Monitoring Consortium Grant 1032144 (M.S.S.) and Collaboration for AIDS Vaccine Discovery Grant OPP1033115 (M.C.N. & J.V.R.). This work was also supported in part by grant # 8 UL1 TR000043 from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program, Center for HIV/AIDS Vaccine Immunology and Immunogen Discovery AI100663-02 (M.C.N.), and National Institute Of Allergy and Infectious Diseases of the NIH Grants AI100148-02 and AI081677-05 (M.C.N. & J.V.R.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. S.B. receives fellowship support from the American Heart Association. M.C.N. is a Howard Hughes Medical Institute Investigator.
Footnotes
Author Contributions. A.H.-S. planned and performed experiments and wrote the manuscript. C.-L.L., F.K., J.A.H., and U.S., helped plan and perform experiments. S.B., L.N., and T.R.E. performed experiments. C.L. and A.G. produced monoclonal antibodies and proteins. M.S.S. performed TZM-bl neutralization measurements. A.T. and J.V.R. planned experiments. M.C.N. planned experiments and wrote the manuscript.
References
- Abela IA, Berlinger L, Schanz M, Reynell L, Gunthard HF, Rusert P, Trkola A. Cell-cell transmission enables HIV-1 to evade inhibition by potent CD4bs directed antibodies. PLoS pathogens. 2012;8:e1002634. doi: 10.1371/journal.ppat.1002634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agosto LM, Zhong P, Munro J, Mothes W. Highly active antiretroviral therapies are effective against HIV-1 cell-to-cell transmission. PLoS pathogens. 2014;10:e1003982. doi: 10.1371/journal.ppat.1003982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aida Y, Pabst MJ. Removal of endotoxin from protein solutions by phase separation using Triton X-114. Journal of immunological methods. 1990;132:191–195. doi: 10.1016/0022-1759(90)90029-u. [DOI] [PubMed] [Google Scholar]
- Alegre ML, Frauwirth KA, Thompson CB. T-cell regulation by CD28 and CTLA-4. In Nat Rev Immunol. 2001:220–228. doi: 10.1038/35105024. [DOI] [PubMed] [Google Scholar]
- Archin NM, Bateson R, Tripathy M, Crooks AM, Yang KH, Dahl NP, Kearney MF, Anderson EM, Coffin JM, Strain MC, et al. HIV-1 Expression within Resting CD4 T-Cells Following Multiple Doses of Vorinostat. In J Infect Dis. 2014:1–26. doi: 10.1093/infdis/jiu155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archin NM, Espeseth A, Parker D, Cheema M, Hazuda D, Margolis DM. Expression of latent HIV induced by the potent HDAC inhibitor suberoylanilide hydroxamic acid. AIDS research and human retroviruses. 2009a;25:207–212. doi: 10.1089/aid.2008.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archin NM, Keedy KS, Espeseth A, Dang H, Hazuda DJ, Margolis DM. Expression of latent human immunodeficiency type 1 is induced by novel and selective histone deacetylase inhibitors. Aids. 2009b;23:1799–1806. doi: 10.1097/QAD.0b013e32832ec1dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archin NM, Liberty AL, Kashuba AD, Choudhary SK, Kuruc JD, Crooks AM, Parker DC, Anderson EM, Kearney MF, Strain MC, et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487:482–485. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch DH, Whitney JB, Moldt B, Klein F, Oliveira TY, Liu J, Stephenson KE, Chang HW, Shekhar K, Gupta S, et al. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature. 2013;503:224–228. doi: 10.1038/nature12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm D, Calvanese V, Dar RD, Xing S, Schroeder S, Martins L, Aull K, Li P-C, Planelles V, Bradner JE, et al. BET bromodomain-targeting compounds reactivate HIV from latency via a Tat-independent mechanism. In cc. 2013a:452–462. doi: 10.4161/cc.23309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm D, Calvanese V, Dar RD, Xing S, Schroeder S, Martins L, Aull K, Li PC, Planelles V, Bradner JE, et al. BET bromodomain-targeting compounds reactivate HIV from latency via a Tat-independent mechanism. Cell cycle. 2013b;12:452–462. doi: 10.4161/cc.23309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsignori M, Pollara J, Moody MA, Alpert MD, Chen X, Hwang KK, Gilbert PB, Huang Y, Gurley TC, Kozink DM, et al. Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. Journal of virology. 2012;86:11521–11532. doi: 10.1128/JVI.01023-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm MA, Wiles MV, Greiner DL, Shultz LD. Generation of improved humanized mouse models for human infectious diseases. Journal of immunological methods. 2014 doi: 10.1016/j.jim.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukrinsky MI, Stanwick TL, Dempsey MP, Stevenson M. Quiescent T lymphocytes as an inducible virus reservoir in HIV-1 infection. Science. 1991;254:423–427. doi: 10.1126/science.1925601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen CK, Laird GM, Durand CM, Siliciano JD, Siliciano RF. New ex vivo approaches distinguish effective and ineffective single agents for reversing HIV-1 latency in vivo. In Nature Publishing Group (Nature Publishing Group) 2014:1–6. doi: 10.1038/nm.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M, Lloyd AL, Nowak MA, Fauci AS. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung AW, Isitman G, Navis M, Kramski M, Center RJ, Kent SJ, Stratov I. Immune escape from HIV-specific antibody-dependent cellular cytotoxicity (ADCC) pressure. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:7505–7510. doi: 10.1073/pnas.1016048108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras X, Schweneker M, Chen CS, McCune JM, Deeks SG, Martin J, Peterlin BM. Suberoylanilide hydroxamic acid reactivates HIV from latently infected cells. The Journal of biological chemistry. 2009;284:6782–6789. doi: 10.1074/jbc.M807898200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar RD, Hosmane NN, Arkin MR, Siliciano RF, Weinberger LS. Screening for noise in gene expression identifies drug synergies. Science. 2014 doi: 10.1126/science.1250220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey RT, Bhat N, Yoder C, Chun TW, Metcalf JA, Dewar R, Natarajan V, Lempicki RA, Adelsberger JW, Miller KD, et al. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci USA. 1999:15109–15114. doi: 10.1073/pnas.96.26.15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MA, Prinjha RK, Dittmann A, Giotopoulos G, Bantscheff M, Chan WI, Robson SC, Chung CW, Hopf C, Savitski MM, et al. Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia. Nature. 2011;478:529–533. doi: 10.1038/nature10509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks SG. HIV: Shock and kill. Nature. 2012;487:439–440. doi: 10.1038/487439a. [DOI] [PubMed] [Google Scholar]
- Denton PW, Olesen R, Choudhary SK, Archin NM, Wahl A, Swanson MD, Chateau M, Nochi T, Krisko JF, Spagnuolo RA, et al. Generation of HIV latency in humanized BLT mice. Journal of virology. 2012;86:630–634. doi: 10.1128/JVI.06120-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhodapkar KM, Kaufman JL, Ehlers M, Banerjee DK, Bonvini E, Koenig S, Steinman RM, Ravetch JV, Dhodapkar MV. Selective blockade of inhibitory Fcgamma receptor enables human dendritic cell maturation with IL-12p70 production and immunity to antibody-coated tumor cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:2910–2915. doi: 10.1073/pnas.0500014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinoso JB, Kim SY, Wiegand AM, Palmer SE, Gange SJ, Cranmer L, O'Shea A, Callender M, Spivak A, Brennan T, et al. Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:9403–9408. doi: 10.1073/pnas.0903107106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybul M, Hidalgo B, Chun TW, Belson M, Migueles SA, Justement JS, Herpin B, Perry C, Hallahan CW, Davey RT, et al. Pilot study of the effects of intermittent interleukin-2 on human immunodeficiency virus (HIV)-specific immune responses in patients treated during recently acquired HIV infection. The Journal of infectious diseases. 2002;185:61–68. doi: 10.1086/338123. [DOI] [PubMed] [Google Scholar]
- Eriksson S, Graf EH, Dahl V, Strain MC, Yukl SA, Lysenko ES, Bosch RJ, Lai J, Chioma S, Emad F, et al. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS pathogens. 2013;9:e1003174. doi: 10.1371/journal.ppat.1003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrantelli F, Buckley KA, Rasmussen RA, Chalmers A, Wang T, Li PL, Williams AL, Hofmann-Lehmann R, Montefiori DC, Cavacini LA, et al. Time dependence of protective post-exposure prophylaxis with human monoclonal antibodies against pathogenic SHIV challenge in newborn macaques. Virology. 2007;358:69–78. doi: 10.1016/j.virol.2006.07.056. [DOI] [PubMed] [Google Scholar]
- Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- Forthal D, Hope TJ, Alter G. New paradigms for functional HIV-specific nonneutralizing antibodies. Current opinion in HIV and AIDS. 2013;8:393–401. doi: 10.1097/COH.0b013e328363d486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forthal DN, Landucci G, Daar ES. Antibody from patients with acute human immunodeficiency virus (HIV) infection inhibits primary strains of HIV type 1 in the presence of natural-killer effector cells. Journal of virology. 2001;75:6953–6961. doi: 10.1128/JVI.75.15.6953-6961.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi RT, Zheng L, Bosch RJ, Chan ES, Margolis DM, Read S, Kallungal B, Palmer S, Medvik K, Lederman MM, et al. The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: a randomized controlled trial. PLoS medicine 7. 2010 doi: 10.1371/journal.pmed.1000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatziioannou T, Evans DT. Animal models for HIV/AIDS research. Nature reviews Microbiology. 2012;10:852–867. doi: 10.1038/nrmicro2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, Lanigan CM, Landucci G, Forthal DN, Parren PW, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- Ho YC, Shan L, Hosmane NN, Wang J, Laskey SB, Rosenbloom DI, Lai J, Blankson JN, Siliciano JD, Siliciano RF. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell. 2013;155:540–551. doi: 10.1016/j.cell.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton HM, Bernett MJ, Peipp M, Pong E, Karki S, Chu SY, Richards JO, Chen H, Repp R, Desjarlais JR, et al. Fc-engineered anti-CD40 antibody enhances multiple effector functions and exhibits potent in vitro and in vivo antitumor activity against hematologic malignancies. Blood. 2010;116:3004–3012. doi: 10.1182/blood-2010-01-265280. [DOI] [PubMed] [Google Scholar]
- Horwitz JA, Halper-Stromberg A, Mouquet H, Gitlin AD, Tretiakova A, Eisenreich TR, Malbec M, Gravemann S, Billerbeck E, Dorner M, et al. HIV-1 suppression and durable control by combining single broadly neutralizing antibodies and antiretroviral drugs in humanized mice. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:16538–16543. doi: 10.1073/pnas.1315295110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi T, Brown C, Azadegan A, Haigwood N, Dimitrov D, Martin MA, Shibata R. Human immunodeficiency virus type 1 neutralizing antibodies accelerate clearance of cell-free virions from blood plasma. Nature medicine. 1999;5:211–216. doi: 10.1038/5576. [DOI] [PubMed] [Google Scholar]
- Jost S, Altfeld M. Control of human viral infections by natural killer cells. Annual review of immunology. 2013;31:163–194. doi: 10.1146/annurev-immunol-032712-100001. [DOI] [PubMed] [Google Scholar]
- Klein F, Halper-Stromberg A, Horwitz JA, Gruell H, Scheid JF, Bournazos S, Mouquet H, Spatz LA, Diskin R, Abadir A, et al. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. In Nature (Nature Publishing Group) 2012a:118–122. doi: 10.1038/nature11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein F, Halper-Stromberg A, Horwitz JA, Gruell H, Scheid JF, Bournazos S, Mouquet H, Spatz LA, Diskin R, Abadir A, et al. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature. 2012b;492:118–122. doi: 10.1038/nature11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krejsgaard T, Kopp K, Ralfkiaer E, Willumsgaard AE, Eriksen KW, Labuda T, Rasmussen S, Mathiesen AM, Geisler C, Lauenborg B, et al. A novel xenograft model of cutaneous T-cell lymphoma. Experimental dermatology. 2010;19:1096–1102. doi: 10.1111/j.1600-0625.2010.01138.x. [DOI] [PubMed] [Google Scholar]
- Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. In J Exp Med. 1995:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon ED, Hurwitz AA, Foster BA, Madias C, Feldhaus AL, Greenberg NM, Burg MB, Allison JP. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:8099–8103. doi: 10.1073/pnas.94.15.8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafeuillade A, Poggi C, Chadapaud S, Hittinger G, Chouraqui M, Pisapia M, Delbeke E. Pilot study of a combination of highly active antiretroviral therapy and cytokines to induce HIV-1 remission. Journal of acquired immune deficiency syndromes. 2001;26:44–55. doi: 10.1097/00126334-200101010-00006. [DOI] [PubMed] [Google Scholar]
- Landovitz R, Curry J. Postexposure Prophylaxis for HIV Infection. In The New England journal of medicine. 2009:1–8. doi: 10.1056/NEJMcp0904189. [DOI] [PubMed] [Google Scholar]
- Lifson JD, Rossio JL, Arnaout R, Li L, Parks TL, Schneider DK, Kiser RF, Coalter VJ, Walsh G, Imming RJ, et al. Containment of Simian Immunodeficiency Virus Infection: Cellular Immune Responses and Protection from Rechallenge following Transient Postinoculation Antiretroviral Treatment. In Journal of virology. 2000:2584–2593. doi: 10.1128/jvi.74.6.2584-2593.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malbec M, Porrot F, Rua R, Horwitz J, Klein F, Halper-Stromberg A, Scheid JF, Eden C, Mouquet H, Nussenzweig MC, et al. Broadly neutralizing antibodies that inhibit HIV-1 cell to cell transmission. The Journal of experimental medicine. 2013;210:2813–2821. doi: 10.1084/jem.20131244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden MD, Kovochich M, Suree N, Shimizu S, Mehta R, Cortado R, Bristol G, An DS, Zack JA. HIV latency in the humanized BLT mouse. Journal of virology. 2012;86:339–347. doi: 10.1128/JVI.06366-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael AJ, Borrow P, Tomaras GD, Goonetilleke N, Haynes BF. The immune response during acute HIV-1 infection: clues for vaccine development. Nature reviews Immunology. 2010;10:11–23. doi: 10.1038/nri2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicodeme E, Jeffrey KL, Schaefer U, Beinke S, Dewell S, Chung CW, Chandwani R, Marazzi I, Wilson P, Coste H, et al. Suppression of inflammation by a synthetic histone mimic. Nature. 2010;468:1119–1123. doi: 10.1038/nature09589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nature reviews Immunology. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- Nischang M, Sutmuller R, Gers-Huber G, Audigé A, Li D, Rochat M-A, Baenziger S, Hofer U, Schlaepfer E, Regenass S, et al. Humanized Mice Recapitulate Key Features of HIV-1 Infection: A Novel Concept Using Long-Acting Anti-Retroviral Drugs for Treating HIV-1. In PloS one. 2012:e38853. doi: 10.1371/journal.pone.0038853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y, Igarashi T, Haigwood NL, Sadjadpour R, Donau OK, Buckler C, Plishka RJ, Buckler-White A, Martin MA. Transfer of neutralizing IgG to macaques 6 h but not 24 h after SHIV infection confers sterilizing protection: implications for HIV-1 vaccine development. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:15131–15136. doi: 10.1073/pnas.2436476100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietzsch J, Gruell H, Bournazos S, Donovan BM, Klein F, Diskin R, Seaman MS, Bjorkman PJ, Ravetch JV, Ploss A, et al. A mouse model for HIV-1 entry. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:15859–15864. doi: 10.1073/pnas.1213409109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins JM, Jurriaans S, van Praag RM, Blaak H, van Rij R, Schellekens PT, ten Berge IJ, Yong SL, Fox CH, Roos MT, et al. Immuno-activation with anti-CD3 and recombinant human IL-2 in HIV-1-infected patients on potent antiretroviral therapy. Aids. 1999;13:2405–2410. doi: 10.1097/00002030-199912030-00012. [DOI] [PubMed] [Google Scholar]
- Quivy V, Adam E, Collette Y, Demonte D, Chariot A, Vanhulle C, Berkhout B, Castellano R, de Launoit Y, Burny A, et al. Synergistic activation of human immunodeficiency virus type 1 promoter activity by NF-kappaB and inhibitors of deacetylases: potential perspectives for the development of therapeutic strategies. Journal of virology. 2002;76:11091–11103. doi: 10.1128/JVI.76.21.11091-11103.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuse S, Calao M, Kabeya K, Guiguen A, Gatot JS, Quivy V, Vanhulle C, Lamine A, Vaira D, Demonte D, et al. Synergistic activation of HIV-1 expression by deacetylase inhibitors and prostratin: implications for treatment of latent infection. PloS one. 2009;4:e6093. doi: 10.1371/journal.pone.0006093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffner T, Sattentau QJ, Duncan CJ. Cell-to-cell spread of HIV-1 and evasion of neutralizing antibodies. Vaccine. 2013;31:5789–5797. doi: 10.1016/j.vaccine.2013.10.020. [DOI] [PubMed] [Google Scholar]
- Shan L, Deng K, Shroff NS, Durand CM, Rabi SA, Yang HC, Zhang H, Margolick JB, Blankson JN, Siliciano RF. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity. 2012;36:491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingai M, Nishimura Y, Klein F, Mouquet H, Donau OK, Plishka R, Buckler-White A, Seaman M, Piatak M, Jr, Lifson JD, et al. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature. 2013;503:277–280. doi: 10.1038/nature12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal A, Kim JT, Balazs AB, Dekel E, Mayo A, Milo R, Baltimore D. Cell-to-cell spread of HIV permits ongoing replication despite antiretroviral therapy. Nature. 2011;477:95–98. doi: 10.1038/nature10347. [DOI] [PubMed] [Google Scholar]
- Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, Margolick JB, Kovacs C, Gange SJ, Siliciano RF. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nature medicine. 2003;9:727–728. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- Siliciano JD, Siliciano RF. HIV-1 eradication strategies: design and assessment. Current opinion in HIV and AIDS. 2013;8:318–325. doi: 10.1097/COH.0b013e328361eaca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Asmal M, Lane S, Permar SR, Schmidt SD, Mascola JR, Letvin NL. Antibody-dependent cell-mediated cytotoxicity in simian immunodeficiency virus-infected rhesus monkeys. Journal of virology. 2011;85:6906–6912. doi: 10.1128/JVI.00326-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C-C, Emau P, Follis K, Beck T, Benveniste R, Bischofberger N, Lifson J, Morton W. Effectiveness of Postinoculation (R)-9-(2-Phosphonylmethoxypropyl)Adenine Treatment for Prevention of Persistent Simian Immunodeficiency Virus SIV Infection Depends Critically on Timing of Initiation and Duration of Treatment. In Journal of virology. 1998:4265. doi: 10.1128/jvi.72.5.4265-4273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C-C, Follis K, Sabo A, Beck T, Grant R, Bischofberger N, Benveniste R, Black R. Prevention of SIV Infection in Macaques by (R)-9-(2-Phosphonylmethoxypropyl)adenine. In Science. 1995:1–3. doi: 10.1126/science.270.5239.1197. [DOI] [PubMed] [Google Scholar]
- West AP, Jr, Scharf L, Scheid JF, Klein F, Bjorkman PJ, Nussenzweig MC. Structural insights on the role of antibodies in HIV-1 vaccine and therapy. Cell. 2014;156:633–648. doi: 10.1016/j.cell.2014.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney JB, Hill AL, Sanisetty S, Penaloza-MacMaster P, Liu J, Shetty M, Parenteau L, Cabral C, Shields J, Blackmore S, et al. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature. 2014 doi: 10.1038/nature13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, Spina CA, Richman DD. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.