FIG. 11.
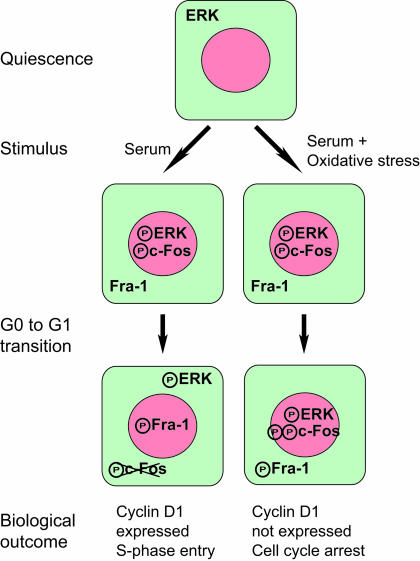
ERK1,2 regulates chromatin trafficking of c-Fos and Fra-1 during cell cycle reentry. We propose a model in which sustained activation of ERK1,2 in response to serum stimulation is required for phosphorylation of c-Fos at C-terminal sites. At the G0-to-G1 transition nuclear ERK1,2 signaling diminishes, leading to dissociation from chromatin and degradation of c-Fos. Termination of nuclear ERK1,2 signaling is followed by recruitment of Fra-1 to chromatin and cyclin D1 expression. Oxidative stress induces cell cycle arrest by prolonging chromatin binding of phospho-ERK, thereby inhibiting the switch from c-Fos to Fra-1 on chromatin required for expression of cyclin D1.