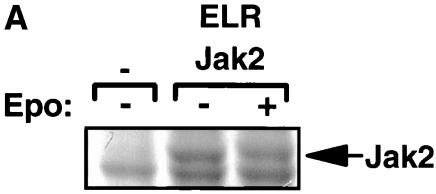
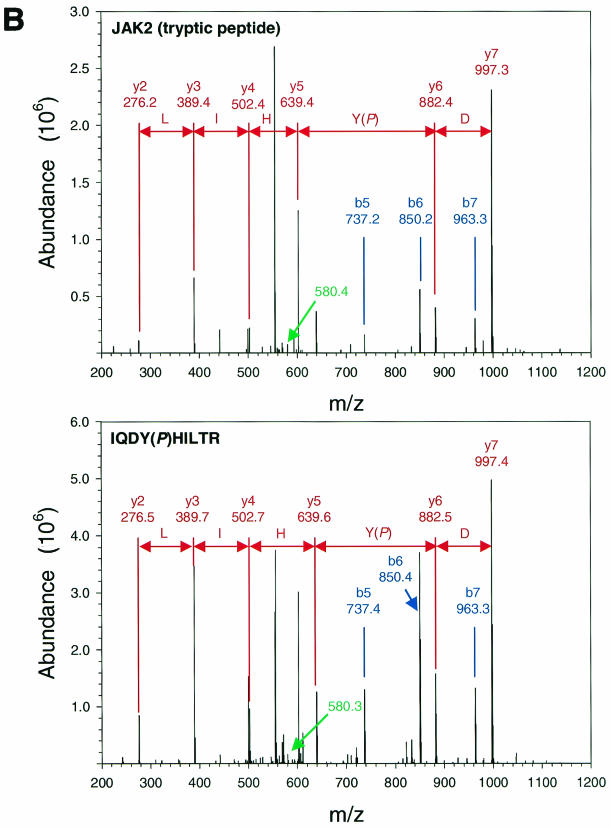
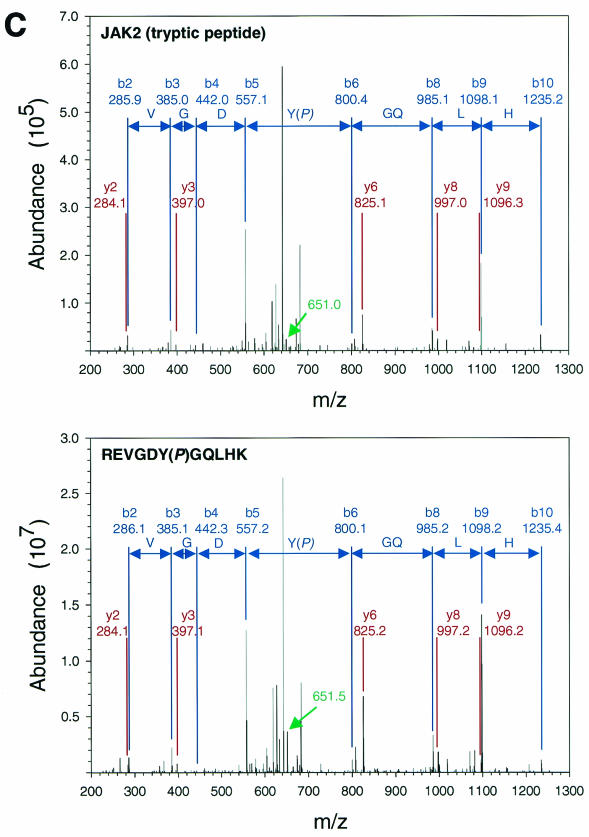
FIG. 1.
Purification and analysis of Jak2 protein. (A) HEK 293 cells were left untransfected or were transfected with the cDNAs for ELR and Jak2, were made quiescent, and were then incubated a further 15 min in the absence (−) or presence (+) of EPO (10 U/ml). Cells were lysed, and lysates were immunoprecipitated with α-Jak2(758). Immunoprecipitated proteins were resolved by SDS-PAGE and detected by staining with Coomassie blue; the relevant region of a representative gel is shown in the panel. The migration of Jak2 protein is indicated to the right of the gel. (B) MS/MS spectra for the Jak2 tryptic peptide phosphorylated at Tyr221 (m/z 620.43; top) and corresponding synthetic peptide, IQDY(P)HILTR (m/z 620.25; bottom). (C) MS/MS spectra for the Jak2 tryptic peptide phosphorylated at Tyr570 (m/z 691.25; top) and corresponding synthetic peptide, REVGDY(P)GQLHK (m/z 691.64; bottom). Sequest assignments of y+ and b+ ions are shown in red and blue, respectively. Fragment ions corresponding to the neutral loss of 40 m/z from these doubly charged precursors are shown in green.