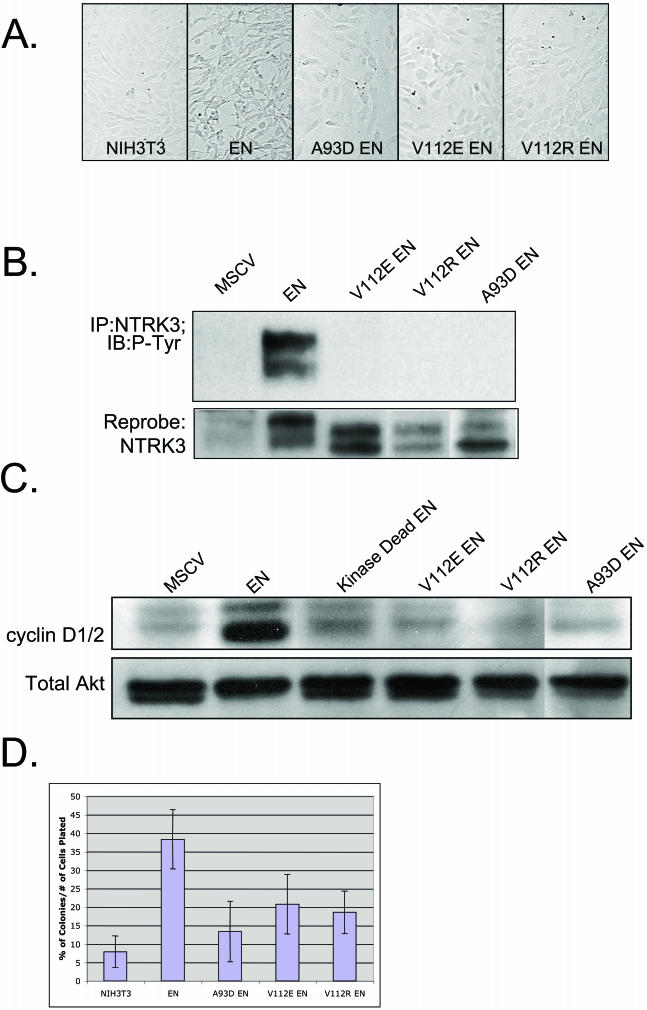
FIG.2.
Characteristics of EN SAM variants with point mutations that disrupt the self-association of the SAM domain. (A) Morphology of NIH 3T3 cells expressing WT EN and EN SAM domain mutants (A93D-EN, V112E-EN, and V112R-EN). WT EN transforms NIH 3T3 cells, whereas the EN SAM domain mutants do not. (B) NTRK3 IP of NIH 3T3 cells expressing WT EN and EN SAM domain mutants probed with antiphosphotyrosine (RC20) and anti-NTRK3 antibodies. Mutant EN SAM proteins are not phosphorylated. Doublets are observed due to an alternative start site within the ETV6 gene. (C) Levels of cyclin D1, detected by Western blotting, in cells expressing WT EN, kinase-dead EN, A93D-EN, V112E-EN, and V112R-EN. WT EN cells express high levels of cyclin D1, but mutant EN cells do not. Equal protein levels were determined with a total AKT antibody. (D) Results of soft agar assay. Cells expressing mutant EN SAM domains form fewer colonies in soft agar than those expressing WT EN.