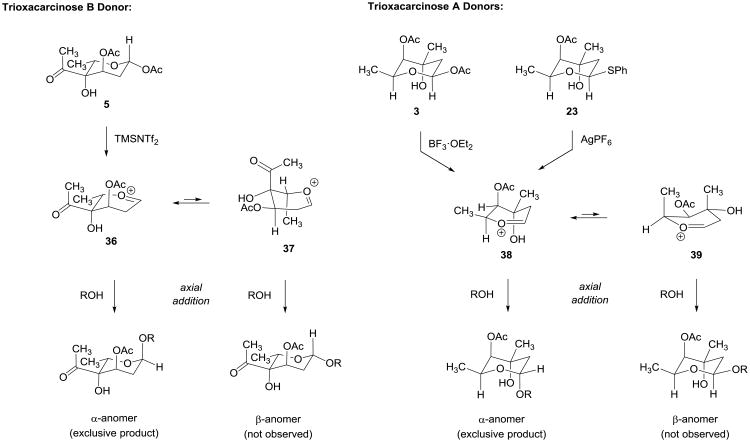
Figure 6. Rationalizations of the stereochemical outcome of glycosylation reactions with trioxacarcinose A and B donors.
Axial addition to the stereoelectronically favored half-chair 36 is relatively less impeded by steric interactions than addition to the alternative conformer 37, offering one rationalization for the high α-selectivities observed in glycosylation reactions of trioxacarcinose B donor 5 (see text for another). Similar considerations apply to explain the highly α-selective couplings of trioxacarcinose A donors 3 and 23.