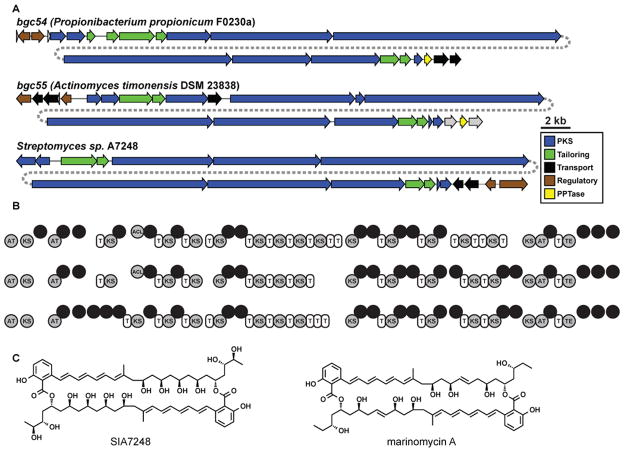
Figure 4. A family of complex PKS BGCs is prevalent in the human oral cavity.
A) Related PKS BGCs in human oral actinobacteria P. propionicum F0230a and A. timonensis DSM 23838 and the marine actinobacterium Streptomyces sp. A7248. The label of each BGC indicates its source organism. B) Domain organization of the three BGCs shown in A (AT, acyltransferase domain; KS, ketosynthase domain; MT, methyltransferase domain; Ox, oxidation domain; ECH, enoyl-CoA hydratase domain; DH, dehydratase domain; KR, ketoreductase domain; ACL, acyl-CoA ligase domain; T, thiolation domain, HCS, HMG-CoA synthase domain; FKB, FkbH-like protein. Note that the domain architecture is remarkably conserved among the three pathways, despite low amino acid sequence identity (~40%). C) Structures of SIA7248, the product of the Streptomyces sp. A7248 BGC shown in A, and the related molecule marinomycin A.