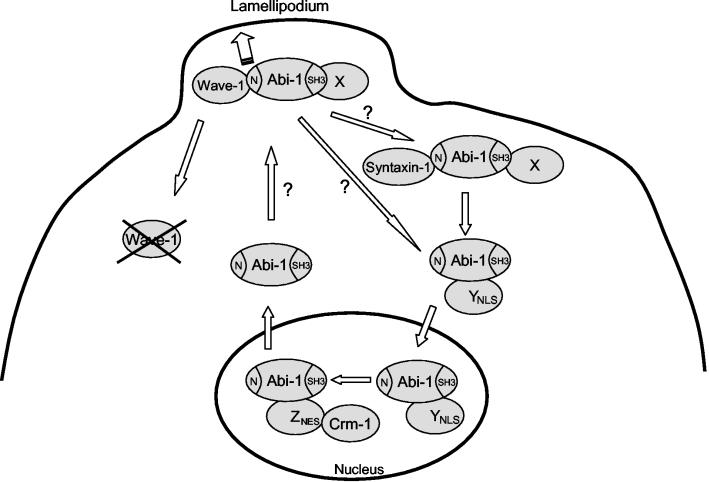
FIG. 9.
Abi-1 localizes to the leading edge of the lamellipodium and undergoes nucleocytoplasmic shuttling. Distinct pools of Abi-1 bind to Wave-1 or Syntaxin-1 in the cytosol, and localization of Abi-1 to the leading edge of the lamellipodium requires the SNARE and WAB domains in the conserved Abi-1 amino terminus. Disruption of the Abi-1-Wave-1 interaction leads to a reduction of Wave-1 protein levels. Moreover, the binding of Wave-1 to Abi-1 contributes to proper localization of Wave-1 to the leading edge of lamellipodia. Abi-1 undergoes nucleocytoplasmic shuttling and accumulates in the nucleus in response to LMB treatment. Localization of Abi-1 to the nucleus in the absence of LMB requires the simultaneous disruption of the SNARE, WAB, and SH3 domains of Abi-1. These findings suggest that multiple protein-protein interactions are involved in retaining Abi-1 in the cytoplasm. The protein (X) that binds to the Abi-1 SH3 domain and contributes to Abi-1 cytoplasmic retention has not yet been identified. While Wave-1 and Syntaxin-1 bind to the WAB and SNARE domains of Abi-1, respectively, other unknown proteins may also bind to these domains and regulate the nucleocytoplasmic distribution of Abi-1. An unknown factor (Y) is required for the translocation of Abi-1 into the nucleus, and another factor (Z) is required for the nuclear export of Abi-1 through the Crm-1-dependent pathway. YNLS, NLS-containing protein; ZNES, NES-containing protein.