Abstract
Background
The Medoff-Cooper Nutritive Sucking Apparatus (M-CNSA) has been used to objectively measure sucking maturation in preterm infants. The M-CNSA is able to accurately detect sucking pressures less than 20 mm Hg, however lower pressure thresholds have not previously been used in research.
Aims
To determine if differences are observed in the number of sucks and maturation in the number of sucks over time when the minimum pressure threshold used to detect a suck is 7 mm Hg compared to 20 mm Hg using the M-CNSA.
Study design
Descriptive.
Subjects
A convenience sample of 171 healthy premature infants born between 29 and 34 weeks gestational period who were part of a larger randomized controlled study.
Outcome measures
The number of sucks detected during weekly five-minute oral feeding observations using 7 mm Hg and 20 mm Hg.
Results
Significantly more sucks were detected using the 7 mm Hg vs 20 mm Hg threshold at all time points. At both pressure thresholds, the mean number of sucks detected during the five minute feeding observation increased over time. The difference in the number of sucks detected at 7 and 20 mm Hg did not change over time (p = 0.50).
Conclusions
Using the lower threshold of 7 mm Hg compared to 20 mm Hg resulted in more sucks detected while consistently measuring improvement in sucking over time. Detection of more sucks and sucks at a lower pressure threshold allows clinicians and researchers to more accurately assess oral feeding skills among premature infants.
Keywords: Sucking skills, Microstructure of infant sucking behaviors, Preterm infants, Nutritive sucking pressures
1. Introduction
Successful oral feeding is a key determinant of a preterm infant’s discharge from the hospital [1]. In order to achieve successful oral feeding, preterm infants must be able to coordinate the components of breathing, sucking, and swallowing [2]. Coordination of these components is a complex task that requires integration, maturation, and coordination of multiple systems within the body [3,4]. Oral feeding is a highly organized behavior and a mature feeding pattern is an indication of neurologic integrity [5–8]. As an infant matures, expected changes in sucking patterns include increases in sucking frequency, maximal sucking pressures, number of sucks per sucking burst, and decreases in time between sucking bursts [9]. Over the past century, multiple methods have been employed to assess maturation of sucking behaviors from the initiation of oral feeding to full oral feeding in preterm infant populations. These methods include observation, checklists such as the Neonatal Oral-Motor Assessment Scale (NOMAS) [10], and video recording feeding bouts to instruments that incorporate pressure transducers to objectively measure various sucking parameters. However, comparison of these early studies is difficult due to variations in instruments and techniques as well as the questionable validity of some of the more subjective methods [7].
In the current research we used the Medoff-Cooper Nutritive Sucking Apparatus (M-CNSA) to objectively measure sucking behaviors in preterm infants during initiation of oral feeding until discharge. In previous research using the M-CNSA, a threshold of 20 mm Hg had been used to identify a nutritive suck. Any changes in intraoral pressure less than 20 mm Hg were considered mouthing rather than true sucking behaviors. With recent advances in signal processing, data analysis software is now able to accurately detect pressure changes which represent real sucking behaviors less than 20 mm Hg. This advent may be especially useful for evaluating the sucking patterns of preterm infants, as maximal sucking pressure has been found to be quite low during the initiation of oral feeding and rises with increasing maturation, feeding experience, and birth weight [9,11,12]. Because all sucking parameters are impacted by the pressure threshold used to define a suck, setting a lower pressure threshold would provide both researchers and clinicians a more accurate assessment of an infant’s ability to feed orally by bottle or breast.
The purpose of this study was to explore whether there is a difference in preterm infants’ observed maturational changes in sucking performance over time when the pressure threshold to detect a nutritive suck is defined as 7 mm Hg vs 20 mm Hg, using a sample of healthy preterm infants from a larger trial. The threshold of 7 mm Hg was chosen based on early sucking research that used a baseline of 7 mm Hg to examine the effects of oral solutions on sucking patterns [13].
2. Methods
2.1. Setting
The infants in this study were admitted to either a level II or level III neonatal intensive care unit (NICU) at one of two community-based hospitals in a large Midwestern city. Recruitment occurred between 2008 and 2011.
2.2. Participants
Participants were otherwise healthy premature infants born between 29 and 34 weeks gestational age (GA) whose mothers had at least two or more social–environmental risk factors, such as low education and poverty. Participants for this study were part of a larger longitudinal, randomized controlled trial investigating a multisensory developmental and participatory guidance intervention for mothers and premature infants. Participants were recruited shortly after birth. Exclusion criteria included infants with congenital anomalies, necrotizing enterocolitis, brain injury, chronic lung disease, or prenatal drug exposure or who were receiving assisted ventilation at the time of enrollment. Mothers were not eligible if they were identified as illicit drug users, HIV positive, or if they were not the legal guardian of their infants.
There were 198 eligible infants enrolled in the larger trial. Additional exclusion criteria for this secondary study pertain to the feeding observations for infants. We excluded seven infants who developed health conditions in the hospital that interfered with feeding, such as pulmonary hypertension, bronchopulmonary dysplasia and conditions which required a transfer to another hospital. An additional three infants were excluded due to an equipment malfunction or departure from protocol during the feeding observation, and four infants were discharged home before a feeding observation could take place.
For this study, feeding observations were also excluded if no sucks were detected at either the 7 mm Hg or 20 mm Hg pressure threshold or if the infant’s measured oral intake was <1 cm3 at the end of the 10 minute feeding, as these feedings were determined to not represent nutritive sucking. We also excluded observations that occurred over 21 days after baseline, as very few infants were still in the hospital at that time and those remaining were not representative of the overall sample of infants due to morbidities or feeding problems that delayed their discharge. Therefore, the final analytic sample size for this study was 171 infants contributing 331 feeding observations.
2.3. Measures
The dependent variables in this study include the number of sucks detected in the five minute observation at 7 mm Hg and at 20 mm Hg, as well as the difference between these two values. The Medoff-Cooper Nutritive Sucking Apparatus (M-CNSA) was used to measure the number of sucks. The M-CNSA is comprised of an ordinary silicone nipple containing an embedded capillary calibrated for metered flow of fluid and a pressure transducer embedded in a second tube. The hardware of the M-CNSA continuously measures negative pressure generated by the infant during nutritive sucking. The pressure signal was fed on-line to an IBM compatible computer, which displayed the pattern of sucks throughout the session and created a sucking record for off-line data analysis, using the Biopac customized software (Goleta, CA). The sucking record was stored digitally using AcqKnowledge 3.9.0 software (Biopac, Goleta, CA). The data for each feeding observation were processed using AcqKnowledge to select the first 5 min of sucking data that was representative of the 10 minute sucking record. During this process, portions of the record during which the nipple was removed from the infant’s mouth were deleted. Other nutritive sucking research has found that a 5 minute recording is adequate for a robust measurement of average pressure [14]. The digital and video recordings were compared for start and finish times of the oral feeding and periods of sucking and resting. The final five-minute segment was analyzed using Matlab 2007a (Natick, MA: The MathWorks) and a custom Matlab subroutine, Suck_Detect 1.1.12 software to generate summary parameters to describe the feeding [15].
To obtain these measures, the Suck_Detect software was implemented twice for each feeding observations, once counting sucks that reached 7 mm Hg of pressure or higher, and a second time counting only sucks that reached the 20 mm Hg pressure threshold. The subtraction of the number of sucks at 20 mm Hg from the number of sucks at 7 mm Hg, therefore, results in a value of zero when all of the babies’ sucks reached 20 mm Hg or higher and a value above zero for babies who had sucks at pressures between 7 and 20 mm Hg.
Infant characteristics were obtained via medical record review and include infant sex, gestational age at birth, birth weight, size for gestational age, plurality, delivery type, clinical site, five-minute Apgar score, infant morbidity measured as a subset of the Problem Oriented Perinatal Risk Assessment System (POPRAS) items that occurred at or near delivery, and postmenstrual age (PMA) and weight at the time of the baseline feeding. PMA at the baseline feeding measurement was calculated by summing the infant’s gestational age at birth and the number of completed weeks of life since birth and grouped into four categories: 31–32, 33, 34, and 35–36 weeks PMA. Maternal race/ethnicity was also obtained via interview with the mother at baseline. Time was measured in two ways for this study. For charts, we display the baseline, second and third weekly feeding observations and for regression models we use the exact day from baseline (day = 0) on which the feeding assessment occurred. For most but not all infants, the second and third feeding observations occurred on days 7 and 14, respectively. Sometimes, however, the schedule was changed to obtain a final observation quickly before hospital discharge, or for other reasons pertaining to the infant, so the second observation occurred on day 6.2 (SD = 1.8) on average and the third observation occurred on day 13.4 (SD = 1.9) on average.
2.4. Procedures
This study was granted institutional review board approval by the university and the two study sites. After confirming eligibility and willingness to participate, written and informed consent was obtained from the mothers of the infants included in this study.
According to the protocol for the larger randomized trial, weekly oral feeding assessments were conducted from initiation of oral feeding through hospital discharge. The baseline feeding assessment was conducted at the first oral feeding when possible, or within a week of initiation of oral feeding, then weekly until hospital discharge. The feeding assessments were conducted during an early morning regularly scheduled feeding, during which collection of sucking data was performed using the M-CNSA. The entire feeding assessment was video recorded. During each feeding assessment, a registered nurse fed the infant using a specialized feeding device from the M-CNSA for a ten minute period. The nipple on the feeding device was used to stimulate the infant’s rooting reflex. When the infant’s mouth was opened, the nipple was introduced into the oral cavity. No other oral stimulation was used during the feeding. Vital signs were monitored throughout the entire feeding period. If the infant demonstrated any signs of distress including bradycardia or breathing difficulties, the nipple was withdrawn from the infant’s mouth and the feeding was paused. At the end of the ten minute feeding assessment, the remainder of the prescribed volume of formula or breast milk was delivered to the infant orally using a regular nipple or via gavage feeding if the infant was demonstrating signs of fatigue. The total volume consumed at each feeding and the route of ingestion were recorded. Sucking pressure was measured and recorded using the M-CNSA as described previously.
2.5. Data analysis
Descriptive statistics were conducted for sample characteristics. Charts were produced to demonstrate the pattern of maturation in sucking over time across the three weekly feeding observations for each PMA group (31–32, 33, 34, and 35–36 weeks) by pressure threshold. The mean number of sucks at each feeding observation was plotted separately for the 7 mm Hg and the 20 mm Hg pressure threshold.
The difference between the number of sucks detected at 7 and 20 mm Hg for each feeding observation and the mean difference was calculated by feeding observation and PMA group. A mixed-effects regression model was employed to examine the effect of time in days on the difference in sucks between the 7 and 20 mm Hg pressure thresholds. The estimate for time in the model was used to assess whether sucking maturation over time would have the same pattern if measured using the 7 or 20 mm Hg pressure threshold. Random subject effects were used to account for the correlations between repeated measures from the same infant. Model selection was performed for random and fixed effects. Infant covariates were chosen for this model using manual backward selection. All analyses were performed using IBM SPSS Statistics version 19.
3. Results
Data were analyzed for 171 infants with a mean GA at birth of 32.6 weeks (SD = 1.5) and a mean birth weight of 1812 g (SD = 355). The sample included 87 female infants (50.9%) and 84 male infants (49.1%). The percentage of infants whose PMA at first oral feeding was 32 or 33 weeks was 10.5% and 21.1%, respectively. See Table 1 for additional characteristics of the sample.
Table 1.
Sample characteristics (n = 171).
| n | % | Mean (SD) | |
|---|---|---|---|
| Infant sex | |||
| Female | 87 | 50.9 | |
| Male | 84 | 49.1 | |
| Maternal race/ethnicity | |||
| African-American | 84 | 49.1 | |
| Latina | 87 | 50.9 | |
| Plurality | |||
| Singleton | 146 | 85.4 | |
| Twin or triplet | 25 | 14.6 | |
| Type of delivery | |||
| Vaginal | 89 | 52.4 | |
| C-section | 81 | 47.6 | |
| Clinical site | |||
| A | 97 | 56.7 | |
| B | 74 | 43.3 | |
| Postmenstrual age (PMA) at baseline feeding observation (weeks) | |||
| 32 | 18 | 10.5 | |
| 33 | 36 | 21.1 | |
| 34 | 62 | 36.3 | |
| 35–36 | 55 | 32.2 | |
| Gestational age at birth | 171 | 32.6 (1.5) | |
| Birth weight (g) | 171 | 1812 (355) | |
| Apgar score at 5 min | 170 | 8.3 (1.0) | |
| Morbidity score (POPRAS) | 165 | 68.2 (18.9) | |
| Weight at baseline (g) | 166 | 1852 (293) | |
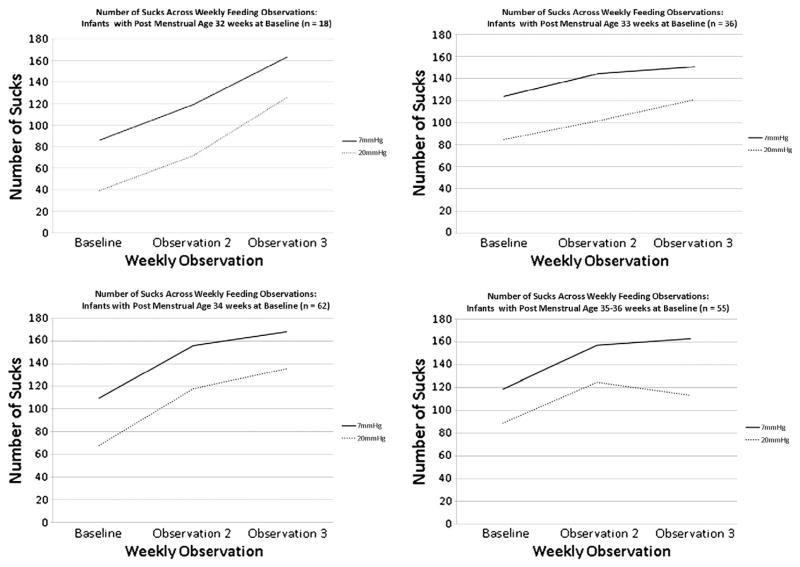
For all PMA groups, the mean number of sucks detected by the M-CNSA at each weekly feeding observation increased over time, regardless of whether the minimum pressure threshold was set at 7 mm Hg or 20 mm Hg (Fig. 1). Table 2 demonstrates the mean difference in the number of sucks detected at the 7 mm Hg compared to the 20 mm Hg threshold by PMA group and observation.
Fig. 1.
Number of sucks across weekly feeding observations.
Table 2.
Mean difference at each time point in number of sucks measured by the 7 mm Hg vs 20 mm Hg pressure threshold, by postmenstrual age (PMA) at baseline feeding.
| PMA at baseline (weeks) | Feeding observation number | Difference in number of sucks (7 mm Hg–20 mm Hg threshold)
|
||||
|---|---|---|---|---|---|---|
| n | Mean | SD | Min | Max | ||
| 32 | 1 | 14 | 46.9 | 37.8 | 6.0 | 135.0 |
| 2 | 12 | 47.4 | 25.2 | 8.0 | 95.0 | |
| 3 | 12 | 37.5 | 19.3 | 14.0 | 69.8 | |
| 4 | 6 | 31.3 | 12.2 | 11.0 | 46.0 | |
| 33 | 1 | 25 | 39.1 | 31.8 | 10.0 | 141.2 |
| 2 | 24 | 42.6 | 31.5 | 16.0 | 149.9 | |
| 3 | 17 | 29.7 | 17.2 | 3.0 | 59.0 | |
| 4 | 5 | 43.2 | 36.8 | 8.9 | 96.0 | |
| 34 | 1 | 42 | 41.5 | 40.3 | 7.0 | 247.8 |
| 2 | 44 | 38.0 | 30.1 | 6.9 | 173.0 | |
| 3 | 24 | 32.6 | 25.2 | 7.0 | 120.6 | |
| 4 | 4 | 36.1 | 19.3 | 15.5 | 57.8 | |
| 35–36 | 1 | 50 | 29.6 | 20.7 | 3.0 | 91.0 |
| 2 | 34 | 32.5 | 19.5 | 4.0 | 92.2 | |
| 3 | 15 | 49.5 | 43.5 | 6.0 | 148.0 | |
| 4 | 3 | 28.1 | 22.2 | 11.0 | 53.1 | |
A random intercept model was found to best fit the outcome of interest, the difference between number of sucks detected at 7 and 20 mm Hg. An interaction between time and PMA group was tested, but it was not significant. In addition, there was no significant time effect on the outcome (p-value = 0.50). These results indicate that the pattern of maturation in sucking is consistent across groups and over time, regardless of the threshold established to detect a nutritive suck. Two covariates are significant in the model. With each increasing week of gestational age at birth, there is a smaller difference in the number of sucks detected at 7 and 20 mm Hg at baseline (β = −2.6). In addition, there is a larger difference in the number of sucks detected between the two pressure thresholds for males compared to females (β = 8.4) (Table 3). These findings indicate that by using the 7 mm Hg threshold, more sucks will be detected, especially for male infants and infants of lower gestational ages, but the pattern of maturation over time is consistent with the pattern observed using the 20 mm Hg threshold.
Table 3.
Final random intercept model for the difference in number of sucks measured using the 7 mm Hg vs 20 mm Hg pressure threshold (n = 171 babies, 331 feeding observations).
| Parameter | Beta estimate | SE | p-Value |
|---|---|---|---|
| Intercept | 119.88 | 35.93 | 0.001 |
| Time (days) | −0.18 | 0.26 | 0.49 |
| Gestational age at birth | −2.64 | 1.09 | 0.02 |
| Infant sex (male vs female) | 8.40 | 3.20 | 0.01 |
4. Discussion
In this sample of healthy preterm infants, using 7 mm Hg as the minimum pressure threshold for detecting a suck compared to 20 mm Hg resulted in the detection of a greater number of sucks while the rate of change in number of sucks overtime remained the same. Thus the 7 mm Hg cutoff for nutritive sucking has the potential to yield more data on additional sucking parameters including suck duration, intersuck width, burst width, and interburst width, in addition to number of sucks. The ability to detect sucks at a lower pressure is especially important when evaluating the emerging sucking patterns of preterm or very low birth weight infants who display immature sucking patterns with low maximal sucking pressure [9,11,16]. The additional data provided by the M-CNSA when the pressure threshold is set to 7 mm Hg will assist researchers and clinicians to more accurately assess the oral feeding skills of these important populations.
Assessment of oral feeding skills and performance is often used by clinicians and researchers to determine readiness for oral feeding and oral feeding ability [17–19]. Lau and Smith’s Oral Feeding Skills (OFS) scale classifies oral feeding skills in preterm infants into one of four levels that include actual feeding skills, fatigue, and endurance, with level one being least mature and level four being most mature [17]. Oral feeding proficiency is determined through quantifying the percentage of volume intake during the first 5 min in relationship to the total volume prescribed and the rate of milk transfer over an entire feeding is measured in milliliters consumed per minute [17]. Thoyre, Shaker, and Pridham developed the Early Feeding Skills (EFS) Assessment, a checklist used to assess the feeding skills of preterm infants [18]. The EFS Assessment is a 36-item scale that uses behavioral and physiologic observations to assess the infant’s oral feeding readiness, oral feeding skill, and oral feeding recovery. Pickler and colleagues used the EFS Assessment to measure the feeding skills of 85 preterm infants pre-discharge and at two weeks post-discharge [19]. The authors found the pre-discharge feeding scores as determined by the EFS Assessment to be predictive of post-discharge feeding scores, demonstrating the usefulness of the EFS Assessment for researchers and caregivers [19].
Objective measures of sucking parameters are also used to quantify maturation of sucking over time. In the present research, we used the M-CNSA to measure the infants’ sucking pressure. Our findings show that sucking maturation can be assessed using 7 mm Hg as a minimum pressure threshold, especially for male infants and premature infants who are still developing their oral sucking capabilities. As an infant’s sucking pattern matures, an increased number of sucks is expected [11]. For each PMA group, the number of sucks increased at each weekly feeding evaluation indicating a maturation of sucking that is consistent with previous research [2,20,21]. This finding persisted when 7 mm Hg, rather than the previously used pressure threshold of 20 mm Hg, was used as the minimum pressure threshold to detect a suck. These findings add to the body of research that has found sucking maturation to be influenced by both age and feeding experience [11,22,23].
The current study is limited by its relatively small sample size of 171 infants. Additionally, this study only included healthy preterm infants. Future research using the M-CNSA in the manner described should include preterm infants at lower GA and infants with morbidities that may influence their feeding maturation and maximal sucking pressure. However, it is likely that using 7 mm Hg as the minimum pressure threshold would help capture more data in these vulnerable populations given that their maximal sucking pressure would be expected to be low.
In summary, using 7 mm Hg as the minimum pressure threshold to detect a suck resulted in more sucks detected while preserving findings related to changes in maturation over time. The greatest difference in the number of sucks detected occurred in infants born at lower gestational ages and in male infants. Detection of more sucks and sucks at a lower pressure threshold offers clinicians and researchers the ability to assess the beginning nutritive sucking capacity of younger and less mature infants.
Acknowledgments
This work was supported by grants from the National Institutes of Child Health and Human Development, the National Institute of Nursing Research (1 R01 HD050738-01A2), and the Harris Foundation. The study sponsors had no role in the study design, in the collection, analysis and interpretation of data, in the writing of the manuscript, or in the decision to submit the manuscript for publication.
We also wish to acknowledge the infants and their families who participated in this research.
Footnotes
Conflict of interest
All authors confirm that they have no financial or personal relationships with other people or organizations that could inappropriately influence their work.
References
- 1.Stark AR, Adamkin DH, Batton DG, Bell EF, Bhutani VK, Denson SE, et al. Hospital discharge of the high-risk neonate: committee on fetus and newborn. Pediatrics. 2008;122(5):1119–26. doi: 10.1542/peds.2008-2174. [DOI] [PubMed] [Google Scholar]
- 2.Amaizu N, Shulman RJ, Schanler RJ, Lau C. Maturation of oral feeding skills in preterm infants. Acta Paediatr. 2008;97(1):61–7. doi: 10.1111/j.1651-2227.2007.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delaney AL, Arvedson JC. Development of swallowing and feeding: prenatal through first year of life. Dev Disabil Res Rev. 2008;14(2):105–17. doi: 10.1002/ddrr.16. [DOI] [PubMed] [Google Scholar]
- 4.Lau C, Smith EO, Schanler RJ. Coordination of suck–swallow and swallow respiration in preterm infants. Acta Paediatr. 2003;92(6):721–7. [PubMed] [Google Scholar]
- 5.Bu’Lock F, Woolridge MW, Baum JD. Development of co-ordination of sucking, swallowing and breathing: ultrasound study of term and preterm infants. Dev Med Child Neurol. 1990;32(8):669–78. doi: 10.1111/j.1469-8749.1990.tb08427.x. Epub 1990/08/01. [DOI] [PubMed] [Google Scholar]
- 6.Medoff-Cooper B, Bilker W, Kaplan JM. Sucking patterns and behavioral state in 1- and 2-day-old full-term infants. J Obstet Gynecol Neonatal Nurs. 2010;39(5):519–24. doi: 10.1111/j.1552-6909.2010.01173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Medoff-Cooper B. Changes in nutritive sucking patterns with increasing gestational age. Nurs Res. 1991;40(4):245–7. Epub 1991/07/01. [PubMed] [Google Scholar]
- 8.Lau C, Alagugurusamy R, Schanler RJ, Smith EO, Shulman RJ. Characterization of the developmental stages of sucking in preterm infants during bottle feeding. Acta Paediatr. 2000;89(7):846–52. Epub 2000/08/16. [PubMed] [Google Scholar]
- 9.Medoff-Cooper B, Bilker W, Kaplan JM. Suckling behavior as a function of gestational age: a cross-sectional study. Infant Behav Dev. 2001;24(1):83–94. [Google Scholar]
- 10.Palmer MM, Crawley K, Blanco IA. Neonatal Oral-Motor Assessment Scale: a reliability study. J Perinatol. 1993;13(1):28–35. Epub 1993/01/01. [PubMed] [Google Scholar]
- 11.Medoff-Cooper B, McGrath JM, Shults J. Feeding patterns of full-term and preterm infants at forty weeks postconceptional age. J Dev Behav Pediatr. 2002;23(4):231–6. doi: 10.1097/00004703-200208000-00007. Epub 2002/08/15. [DOI] [PubMed] [Google Scholar]
- 12.Wrotniak BH, Stettler N, Medoff-Cooper B. The relationship between birth weight and feeding maturation in preterm infants. Acta Paediatr. 2009;98(2):286–90. doi: 10.1111/j.1651-2227.2008.01111.x. Epub 2008/11/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaye H. Effect of variations of oral experience upon suckle. In: Bosma JF, editor. Oral sensation and perception: the mouth of the infant. Springfield, IL: Charles C Thomas; 1972. pp. 261–92. [Google Scholar]
- 14.Medoff-Cooper B. Nutritive sucking research: from clinical questions to research answers. J Perinat Neonatal Nurs. 2005;19(3):265–72. doi: 10.1097/00005237-200507000-00013. Epub 2005/08/18. [DOI] [PubMed] [Google Scholar]
- 15.Adnani F. Suck_detect handbook. Richmond, VA: University of Pennsylvania; 2008. [Google Scholar]
- 16.Matsubara M, Tamura Y, Ruchala P. Analysis of nutritive sucking function in very low and extremely low birthweight infants in Japan: a pilot study. Jpn J Nurs Sci. 2005;2(1):3–7. [Google Scholar]
- 17.Lau C, Smith EO. A novel approach to assess oral feeding skills of preterm infants. Neonatology. 2011;100(1):64–70. doi: 10.1159/000321987. Epub 2011/01/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thoyre SM, Shaker CS, Pridham KF. The early feeding skills assessment for preterm infants. Neonatal Netw. 2005;24(3):7–16. doi: 10.1891/0730-0832.24.3.7. Epub 2005/06/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pickler RH, Reyna BA, Griffin JB, Lewis M, Thompson AM. Changes in oral feeding in preterm infants two weeks after hospital discharge. Newborn Infant Nurs Rev. 2012;12(4):202–6. doi: 10.1053/j.nainr.2012.09.012. Epub 2012/11/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cunha M, Barreiros J, Gonçalves I, Figueiredo H. Nutritive sucking pattern—from very low birth weight preterm to term newborn. Early Hum Dev. 2009;85(2):125–30. doi: 10.1016/j.earlhumdev.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Pickler RH, Best AM, Reyna BA, Gutcher G, Wetzel PA. Predictors of nutritive sucking in preterm infants. J Perinatol. 2006;26(11):693–9. doi: 10.1038/sj.jp.7211590. Epub 2006/09/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howe TH, Sheu CF, Hinojosa J, Lin J, Holzman IR. Multiple factors related to bottle-feeding performance in preterm infants. Nurs Res. 2007;56(5):307–11. doi: 10.1097/01.NNR.0000289498.99542.dd. [DOI] [PubMed] [Google Scholar]
- 23.Pickler RH, Best A, Crosson D. The effect of feeding experience on clinical outcomes in preterm infants. J Perinatol. 2009;29(2):124–9. doi: 10.1038/jp.2008.140. Epub 2008/10/03. [DOI] [PMC free article] [PubMed] [Google Scholar]