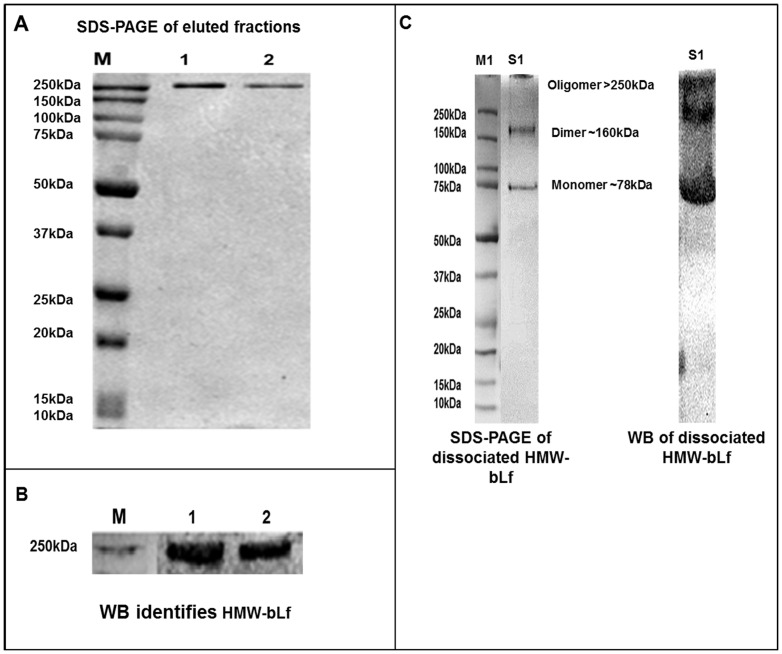
Figure 1. Purification and analysis of components.
A) SDS-PAGE analysis of purified HMW-bLf indicating the presence of pure ≥250 kDa protein in elutes (lanes 1 and 2). B) The purified protein was confirmed to be bLf through Western blotting using anti-bLf specific antibody. C) Dissociation of HMW-bLf in 1 M NaCl into the dimeric (∼160 kDa) and monomeric (∼78 kDa) forms (lane S1). These were confirmed to be bLf bands by Western blot for the same dissociated sample (S1). The absence of any other lower bands and the detection of all the constituent bands by anti-bLf specific antibody indicate that HMW-bLf is an oligomer formed by the interactions of monomeric bLf molecules.