Abstract
Background
Migraine is a highly disabling condition for the individual and also has wide‐reaching implications for society, healthcare services, and the economy. Sumatriptan is an abortive medication for migraine attacks, belonging to the triptan family. Subcutaneous administration may be preferable to oral for individuals experiencing nausea and/or vomiting
Objectives
To determine the efficacy and tolerability of subcutaneous sumatriptan compared to placebo and other active interventions in the treatment of acute migraine attacks in adults.
Search methods
We searched Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, online databases, and reference lists for studies through 13 October 2011.
Selection criteria
We included randomised, double‐blind, placebo‐ and/or active‐controlled studies using subcutaneous sumatriptan to treat a migraine headache episode, with at least 10 participants per treatment arm.
Data collection and analysis
Two review authors independently assessed trial quality and extracted data. We used numbers of participants achieving each outcome to calculate relative risk (or 'risk ratio') and numbers needed to treat to benefit (NNT) or harm (NNH) compared to placebo or a different active treatment.
Main results
Thirty‐five studies (9365 participants) compared subcutaneous sumatriptan with placebo or an active comparator. Most of the data were for the 6 mg dose. Sumatriptan surpassed placebo for all efficacy outcomes. For sumatriptan 6 mg versus placebo the NNTs were 2.9, 2.3, 2.2, and 2.1 for pain‐free at one and two hours, and headache relief at one and two hours, respectively, and 6.1 for sustained pain‐free at 24 hours. Results for the 4 mg and 8 mg doses were similar to the 6 mg dose, with 6 mg significantly better than 4 mg only for pain‐free at one hour, and 8 mg significantly better than 6 mg only for headache relief at one hour. There was no evidence of increased migraine relief if a second dose of sumatriptan 6 mg was given after an inadequate response to the first.
Relief of headache‐associated symptoms, including nausea, photophobia, and phonophobia, was greater with sumatriptan than with placebo, and use of rescue medication was lower with sumatriptan than placebo. For the most part, adverse events were transient and mild and were more common with sumatriptan than placebo.
Sumatriptan was compared directly with a number of active treatments, including other triptans, acetylsalicylic acid plus metoclopramide, and dihydroergotamine, but there were insufficient data for any pooled analyses.
Authors' conclusions
Subcutaneous sumatriptan is effective as an abortive treatment for acute migraine attacks, quickly relieving pain, nausea, photophobia, phonophobia, and functional disability, but is associated with increased adverse events.
Plain language summary
Sumatriptan (subcutaneous route of administration) for acute migraine attacks in adults
Sumatriptan is one of the triptan family of drugs used to treat migraine attacks. It is available as a subcutaneous injection, and this route of administration may be preferable for individuals experiencing nausea and/or vomiting, or needing fast relief. This review found that a single subcutaneous dose was effective in relieving migraine headache pain and associated symptoms of nausea, sensitivity to light, and sensitivity to sound. Pain was reduced from moderate or severe to no pain by two hours in almost 6 in 10 people (59%) taking sumatriptan 6 mg, compared with about 1 in 7 (15%) taking placebo, and reduced from moderate or severe to no worse than mild pain by two hours in almost 8 in 10 people (79%) taking sumatriptan compared with about 3 in 10 (31%) taking placebo. Subcutaneous sumatriptan was fast‐acting, and the majority of people experiencing pain relief had done so by one hour. About 3 in 10 (31%) people had freedom from pain at two hours which was sustained during the 24 hours postdose without the use of rescue medication, compared with about 1 in 7 (15%) with placebo. In addition to relieving headache pain, sumatriptan also relieved symptoms of nausea and sensitivity to light and sound by two hours in about half of those who took it, compared with about one‐third of those taking placebo. Adverse events, most of which were of short duration and mild or moderate in severity, were more frequent with sumatriptan than with placebo.
Background
Description of the condition
Migraine is a common, disabling headache disorder, with considerable social and economic impact (Hazard 2009). Recent reviews found a one‐year prevalence of 15% for adults in European countries (Stovner 2010) and 13% for all ages in the US (Victor 2010). Migraine is more prevalent in women than in men (by a factor of two to three), and in the age range 30 to 50 years.
The International Headache Society (IHS) classifies two major subtypes. Migraine without aura is the most common subtype. It is characterised by attacks lasting 4 to 72 hours that are typically of moderate to severe pain intensity, unilateral, pulsating, aggravated by normal physical activity, and associated with nausea and/or photophobia and phonophobia. Migraine with aura is characterised by reversible focal neurological symptoms that develop over a period of 5 to 20 minutes and last for less than 60 minutes, followed by headache with the features of migraine without aura. In some cases the headache may lack migrainous features or be absent altogether (IHS 2004).
A recent large prevalence study in the US found that over half of migraineurs had severe impairment or required bed rest during attacks. Despite this high level of disability and a strong desire for successful treatment, only a proportion of migraine sufferers seek professional advice for the treatment of attacks. The majority were not taking any preventive medication, although one‐third met guideline criteria for offering or considering it. Nearly all (98%) migraineurs used acute treatments for attacks, with 49% using over‐the‐counter (OTC) medication only, 20% using prescription medication, and 29% using both. OTC medication included aspirin, other non‐steroidal anti‐inflammatory drugs (NSAIDs), paracetamol (acetaminophen), and paracetamol with caffeine (Bigal 2008; Diamond 2007; Lipton 2007). Similar findings have been reported from other large studies in France and Germany (Lucas 2006; Radtke 2009).
The significant impact of migraine with regard to pain, disability, social functioning, quality of relationships, emotional well‐being, and general health (Edmeads 1993; Osterhaus 1994; Solomon 1997) results in a huge burden for the individual, health services, and society (Clarke 1996; Ferrari 1998; Hazard 2009; Hu 1999; Solomon 1997). The annual US economic burden relating to migraine, including missed days of work and lost productivity, is USD 14 billion (Hu 1999). Thus successful treatment of acute migraine attacks not only benefits patients by reducing their disability and improving health‐related quality of life, but also reduces the need for healthcare resources and increases economic productivity (Jhingran 1996; Lofland 1999).
Description of the intervention
The symptomatic treatment of migraine advanced significantly with the development of the triptan class of drugs, of which sumatriptan was the first, in 1991. It is available as a standard oral tablet, nasal spray, rectal suppositories, and subcutaneous (sc) injection. The subcutaneous formulation is available only by prescription. Generic (non‐proprietary) formulations are becoming available. The subcutaneous formulation may be particularly useful for individuals who experience severe nausea or vomiting with their attacks, or who need fast relief. In England in 2010 there were over 910,000 prescriptions for sumatriptan in primary care, of which 54,900 were for the subcutaneous injection (PCA 2011).
In order to establish whether sumatriptan is an effective treatment at a specified dose in acute migraine attacks, it is necessary to study its effects in circumstances that permit detection of pain relief. Such studies are carried out in individuals with established pain of moderate to severe intensity, using single doses of the interventions. Participants who experience an inadequate response with either placebo or active treatment are permitted to use rescue medication, and the intervention is considered to have failed in those individuals. In clinical practice, however, individuals would not normally wait until pain is of at least moderate severity, and may take a second dose of medication if the first dose does not provide adequate relief. Once efficacy is established in studies using single doses in established pain, further studies may investigate different treatment strategies and patient preferences.
How the intervention might work
Sumatriptan is a 5‐HT1 agonist, selectively targeting the 5‐HT (serotonin) 1B and 1D receptors. It has three putative mechanisms of therapeutic action (Ferrari 2002; Goadsby 2007):
vasoconstriction of dilated meningeal blood vessels;
inhibition of the release of vasoactive neuropeptides from perivascular trigeminal sensory neurons;
reduction of pain signal transmission in the trigeminal dorsal horn.
It is used for acute treatment, having no efficacy in preventing future attacks. Oral sumatriptan suffers from poor bioavailability due to metabolism in the gastrointestinal tract before reaching the bloodstream and target arteries. An early suggestion was that injecting the drug subcutaneously would lead to greater efficacy and faster onset of effect.
Why it is important to do this review
Sumatriptan was the first marketed triptan, is by far the most used triptan worldwide, and has become the standard against which new acute migraine treatments are compared. An earlier Cochrane review of oral sumatriptan for acute migraine headaches searched for studies to the end of 2001 (McCrory 2003) and included comparisons with placebo, no intervention, other drug treatments, and behavioural or physical therapies. More studies have been published since that time, and an update is needed to include and evaluate the data from these. We decided to include all routes of administration in the update, and to limit comparators to placebo and other pharmacological interventions. Owing to the very large amount of information now available, particularly for the oral formulation, we carried out separate reviews for each route of administration (Derry 2012a; Derry 2012b; Derry 2012c; Derry 2012d), together with an overview of all routes of administration (Derry (forthcoming)). These sumatriptan reviews form part of a larger series of reviews planned for acute treatments for migraine attacks.
The present review considers subcutaneous administration only, for which a significant body of evidence exists. This is the most costly formulation of sumatriptan, and is likely to benefit primarily those who experience severe nausea and vomiting, and those needing fast relief; its place in the overall spectrum of migraine therapies needs to be evaluated with these considerations in mind. In addition to the original branded subcutaneous sumatriptan, generic versions and needle‐free injection devices that deliver sumatriptan beneath the skin's surface using compressed gas have recently become available and need to be addressed.
Objectives
The objective of this review is to determine the efficacy and tolerability of subcutaneous sumatriptan compared to placebo and other active interventions in the treatment of acute migraine attacks in adults.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised, double‐blind, placebo‐ and/or active‐controlled studies using subcutaneous sumatriptan to treat a migraine headache episode. Studies had to have a minimum of 10 participants per treatment arm and report dichotomous data for at least one of the outcomes specified below. We accepted studies reporting treatment of consecutive headache episodes if outcomes for the first, or each, episode were reported separately. Cross‐over studies were accepted if there was adequate washout (≥ 48 hours) between treatments.
Types of participants
Studies enrolled adults (at least 18 years of age) with migraine. We used the definition of migraine specified by the International Headache Society (IHS 1988; IHS 2004), although we accepted diagnostic criteria equivalent to those of IHS 1988, where a specific reference was not provided. There were no restrictions on migraine frequency, duration, or type (with or without aura). Participants taking stable prophylactic therapy to reduce migraine frequency were accepted; where reported, details on the prophylactic therapy prescribed or allowed are provided in the Characteristics of included studies table.
Types of interventions
We included studies in which self administered subcutaneous sumatriptan was used to treat a migraine headache episode. There were no restrictions on dose, dosing regimen (e.g. single dose versus optional second dose), or timing of the first dose in relation to headache intensity (e.g. taking the first dose when pain was of moderate or severe intensity versus when pain was only mild).
A placebo comparator is essential to demonstrate that sumatriptan is effective in this condition. Active‐controlled trials without a placebo were considered as secondary evidence. We excluded studies designed to demonstrate prophylactic efficacy in reducing the number or frequency of migraine headaches.
Types of outcome measures
Primary outcomes
In selecting the main outcome measures for this review, we considered scientific rigour, availability of data, and patient preferences (Lipton 1999). Patients with acute migraine headaches have rated complete pain relief, no headache recurrence, rapid onset of pain relief, and no side effects as the four most important outcomes (Lipton 1999).
In view of these patient preferences, and in line with the guidelines for controlled trials of drugs in migraine issued by the IHS (IHS 2000), we considered the following primary outcomes:
pain‐free at one and two hours, without the use of rescue medication;
reduction in headache pain ('headache relief') at one and two hours (pain reduced from moderate or severe to none or mild without the use of rescue medication);
sustained pain‐free during the 24 hours postdose (pain‐free within two hours, with no use of rescue medication or recurrence of moderate to severe pain within 24 hours);
sustained headache relief during the 24 hours postdose (headache relief at two hours, sustained for 24 hours, with no use of rescue medication or a second dose of study medication).
Pain intensity or pain relief had to be measured by the patient (not the investigator or carer). We accepted the following pain measures for the primary outcomes:
pain intensity: four‐point categorical scale, with wording equivalent to none, mild, moderate, and severe; or 100 mm visual analogue scale (VAS);
pain relief: five‐point categorical scale, with wording equivalent to none, a little, some, a lot, complete; or 100 mm VAS.
All included studies used one or more of these standard scales and reported outcomes as defined above. We considered only data obtained directly from the patient.
Secondary outcomes
Secondary outcomes considered were:
use of rescue medication;
participants with any adverse event during the 24 hours postdose;
participants with particular adverse events during the 24 hours postdose;
withdrawals due to adverse events;
headache‐associated symptoms: relief and/or presence at two hours;
functional disability: relief and/or presence at two hours.
Although recurrence of headache is perceived to be a problem with triptan medication, we chose not to analyse this outcome because of variation in the definition of 'recurrence' and poor reporting, such that it is often unclear whether the result is reported as a proportion of the whole treatment group or only of those who experienced headache relief at two hours. Furthermore, because recurrence is dependent upon first experiencing headache relief at two hours ‐ an outcome that varies across different treatment groups ‐ interpretation of the result is difficult. We believe that the outcome of sustained headache relief at 24 hours qualitatively provides the same information to patients, but in a more rigorous and intuitive way.
Definitions of important terms, including all measured outcomes, are provided in Appendix 1.
Search methods for identification of studies
Electronic searches
We searched the following databases:
the Cochrane Central Register of Controlled Trials (CENTRAL) (2011, Issue 10);
MEDLINE (via OVID) (to 13 October 2011);
EMBASE (via OVID) (to 13 October 2011);
Oxford Pain Relief Database (Jadad 1996a).
See Appendix 2, Appendix 3, and Appendix 4 for the search strategies for MEDLINE, EMBASE, and CENTRAL, respectively. There were no language restrictions.
Searching other resources
We searched reference lists of retrieved studies and review articles for additional studies. We also searched online clinical trials databases (www.gsk‐clinicalstudyregister.com and www.clinicaltrials.gov). We made a written request for information about both published and unpublished data from the manufacturer of sumatriptan (GlaxoSmithKline), but no additional studies were identified. We did not search grey literature and abstracts.
Data collection and analysis
Selection of studies
Two review authors independently carried out the searches and selected studies for inclusion. We viewed titles and abstracts of all studies identified by electronic searches on screen and excluded any that clearly did not satisfy the inclusion criteria. We read full copies of the remaining studies to identify those suitable for inclusion. Disagreements were settled by discussion with a third review author.
Data extraction and management
Two review authors independently extracted data from included studies using a standard data extraction form. Disagreements were settled by discussion with a third review author. One author entered data into RevMan 5.1 (RevMan 2011).
Assessment of risk of bias in included studies
We assessed methodological quality using the Oxford Quality Score (Jadad 1996b).
The scale is used as follows:
Is the study randomised? If yes, give one point.
Is the randomisation procedure reported and is it appropriate? If yes, add one point; if no, deduct one point.
Is the study double‐blind? If yes, add one point.
Is the double‐blind method reported and is it appropriate? If yes, add one point; if no, deduct one point.
Are the reasons for patient withdrawals and dropouts described? If yes, add one point.
The scores for each study are reported in the Characteristics of included studies table.
We also completed a 'Risk of bias' table for each study, using assessments of random sequence generation, allocation concealment, blinding, and study size.
Measures of treatment effect
We used relative risk (or 'risk ratio', RR) to establish statistical difference. We used numbers needed to treat (NNT) and pooled percentages as absolute measures of benefit or harm.
We used the following terms to describe adverse outcomes in terms of harm or prevention of harm:
When significantly fewer adverse outcomes occurred with sumatriptan than with control (placebo or active) we used the term the number needed to treat to prevent one event (NNTp).
When significantly more adverse outcomes occurred with sumatriptan compared with control (placebo or active) we used the term the number needed to harm or cause one event (NNH).
Unit of analysis issues
We accepted randomisation at the individual patient level only.
Dealing with missing data
The most likely source of missing data was in cross‐over studies. Where this might be problematic (e.g. where data were missing for > 10% of participants), we used only first‐period data, where available. In all cases (cross‐over or parallel‐group) where there were substantial missing data we commented on this and performed sensitivity analyses to investigate their effect.
Assessment of heterogeneity
We assessed heterogeneity of response rates using L'Abbé plots, a visual method for assessing differences in results of individual studies (L'Abbé 1987).
Assessment of reporting biases
We assessed publication bias by examining the number of participants in trials with zero effect (relative risk of 1.0) needed for the point estimate of the NNT to increase beyond a clinically useful level (Moore 2008). In this case, we specified a clinically useful level as a NNT of ≥ 8 for pain‐free at two hours, and a NNT of ≥ 6 for headache relief at two hours.
Data synthesis
We analysed studies using a single dose of sumatriptan in established pain of at least moderate intensity separately from studies in which medication was taken before pain was well established or in which a second dose of medication was permitted.
We calculated effect sizes and combined data for analysis only for comparisons and outcomes where there were at least two studies and 200 participants (Moore 1998). We calculated relative risk of benefit or harm with 95% confidence intervals (CIs) using a fixed‐effect model (Morris 1995). We calculated NNT, NNTp, and NNH with 95% CIs using the pooled number of events by the method of Cook and Sackett (Cook 1995). We assumed a statistically significant difference from control when the 95% CI of the relative risk of benefit or harm did not include the number one.
We determined significant differences between NNT, NNTp, and NNH for different doses of active treatment, or between groups in the sensitivity analyses, using the z test (Tramer 1997).
We describe data from comparisons and outcomes with only one study or fewer than 200 participants in the summary tables and text where appropriate for information and comparison, but we did not analyse these data quantitatively.
Subgroup analysis and investigation of heterogeneity
We analysed different doses and treatment regimens separately. No further subgroup analysis was planned.
Sensitivity analysis
We planned sensitivity analysis for study quality (Oxford Quality Score of 2 versus 3 or more) and for migraine type (with aura versus without aura). A minimum of two studies and 200 participants were required for any sensitivity analysis.
Results
Description of studies
Included studies
Thirty‐five studies (32 publications) fulfilled the inclusion criteria for this review; 30 were published in full peer‐reviewed journals (Akpunonu 1995; Bates 1994; Bousser 1993; Cady 1991 Study 1 and Study 2; Cady 1993; Cady 1998; Dahlof 1992; Dahlof 1998; Diener 1999; Diener 2001; Facchinetti 1995; Ferrari 1991; Gross 1994; Henry 1993; Jensen 1995; Mathew 1992; Mushet 1996 Study 1 and Study 2; Pfaffenrath 1991; Russell 1994; Sang 2004; Schulman 2000; Thomson 1993; Visser 1992; Wendt 2006; Winner 1996; Winner 2006 Study 1 and Study 2), and five were available as Results Summaries on the manufacturer's website (S2BL99; S2BM03; S2BS78; SUM40286; SUM40287). These studies provided data on 9365 participants.
All of the included studies recruited adult participants only, with the majority (23/35) recruiting participants between 18 and 65 years of age (mean ages ranged from 37 to 45 years), and the remainder ranging from a 50‐year maximum age to no upper limit on age. The majority of participants were female (55% to 100%) and had a diagnosis of migraine without aura (61% to 100%). Most studies required participants to have had at least a 6‐ or 12‐month history of migraine attacks meeting IHS (or equivalent) diagnostic criteria (IHS 1988; IHS 2004) before screening, although five studies (Henry 1993; Jensen 1995; Mathew 1992; Thomson 1993; Wendt 2006) made no specific requirement for length of migraine history, and one (Russell 1994) had 90% of participants with IHS criteria in a post‐treatment analysis. Five studies required participants to discontinue any prophylactic medication at least two weeks before receiving study medication, while 14 studies allowed stable prophylactic medications (often excluding monoamine oxidase inhibitors, methysergide and ergotamine or ergotamine‐containing medications), and the remaining 16 studies did not report on prophylaxis. Twenty studies restricted participants from taking study medication within a defined time period of other acute migraine medications. This was most often 24 hours for any opiate, ergotamine, or triptan use, and six hours for any simple analgesics or antiemetics. The remaining 14 studies did not report on restricted acute migraine medications.
Participants were generally excluded for: pregnancy or breast‐feeding, inadequate contraception, confirmed or suspected cardiovascular or cerebrovascular disease (particularly history of ischaemic heart disease), uncontrolled hypertension (diastolic ≥ 95 mmHg or systolic ≥ 160 mmHg), current or past drug abuse, psychiatric illness, epilepsy, hepatic disease, Raynaud's syndrome, and/or opthalmoplegic, basilar or hemiplegic migraine. In addition 14 studies excluded participants if they had previously taken sumatriptan: some limited this exclusively to subcutaneous sumatriptan and others excluded participants who had any experience with sumatriptan. Two studies (SUM40286; SUM40287) required participants to have successfully treated an attack with a 5HT1 agonist in the past, but never to have used a subcutaneous formulation. One study (S2BM03) actually required participants to have regularly used sumatriptan for at least six months before study entry and to experience recurrence of headache in 50% or more of their treated attacks.
The baseline headache intensity at which study medication was administered was largely consistent amongst the included studies, with the majority (25/35) administering the study drug when migraine headache pain was of moderate or severe intensity. Of the remaining studies, one (Bates 1994) required participants to administer medication at the onset of aura, one (S2BM03) at the onset of migraine, and one (S2BS78) at the first sign of headache pain. Seven studies did not report the baseline headache intensity at which study medication was administered. Despite this variability in instruction on when to medicate, all 10 of these studies were dominated by participants with moderate or severe migraine attacks at the time of dosing, and all except one (S2BS78) provided data based on this population specifically. S2BS78 reported on a mixed population of participants treating either mild intensity headaches or moderate and severe intensity headaches, and failed to provide specific data for either population. Given the clinical heterogeneity between these two populations of participants, this study did not provide any data toward efficacy analyses.
Most of the included studies used a parallel‐group design (28/35), treating a single migraine attack (25/35). Of those studies treating multiple attacks, most (7/10) treated two separate attacks. The response of headaches to study treatment was measured using a standard four‐point pain intensity scale in all 35 studies. The majority of the studies (27/35) reported at least one IHS‐preferred outcome (IHS 2000); seven studies (Akpunonu 1995; Cady 1998; Jensen 1995; Russell 1994; S2BS78; Thomson 1993; Visser 1992) provided data for secondary outcomes only. Just over half of the studies (19/35) offered participants the option of a second dose of study medication if either the initial response had been inadequate, or if the participant experienced recurrence (defined as a relapse of moderate or severe intensity headache after an initial response), (13 studies), or to treat recurrence alone (six studies). All studies reported allowing rescue medication (often excluding ergotamine or ergotamine‐derivatives) if the response to study treatment was insufficient after a defined time period. This time period varied between studies, with some studies allowing the use of some form of rescue medication 0.5, 1, 1.5, 2, and 4 hours after initial dosing (1, 3, 2, 20 and 1 study, respectively), while others allowed rescue medication at either one or two hours after administration of a second dose of study medication (five and three studies, respectively). In some cases rescue medication was available to treat recurrence as well as inadequate response, but most studies did not address this question specifically.
Twenty‐eight studies used only a placebo comparator, three studies used only active comparators, and four used both active and placebo comparators. All of the included studies used a needle‐based delivery system; no studies reporting efficacy results from needle‐free injection systems were found. The 35 studies reported on 18 different treatment comparisons:
Sumatriptan 1 mg versus placebo (Mathew 1992; Visser 1992).
Sumatriptan 2 mg versus placebo (Mathew 1992; Visser 1992).
Sumatriptan 3 mg versus placebo (Mathew 1992; Visser 1992).
Sumatriptan 4 mg versus placebo (Mathew 1992; Thomson 1993; Wendt 2006).
Sumatriptan 6 mg versus placebo (Akpunonu 1995; Bates 1994; Bousser 1993; Cady 1991 Study 1 and Study 2; Cady 1993; Cady 1998; Dahlof 1998; Diener 1999; Diener 2001; Facchinetti 1995; Ferrari 1991; Gross 1994; Henry 1993; Jensen 1995; Mathew 1992; Mushet 1996 Study 1 and Study 2; Pfaffenrath 1991; Russell 1994; S2BM03; S2BS78; Sang 2004; Schulman 2000; SUM40286; SUM40287; Winner 2006 Study 1 and Study 2).
Sumatriptan 6 mg versus subcutaneous naratriptan 0.5 mg (Dahlof 1998).
Sumatriptan 6 mg versus subcutaneous naratriptan 1 mg (Dahlof 1998).
Sumatriptan 6 mg versus subcutaneous naratriptan 2.5 mg (Dahlof 1998).
Sumatriptan 6 mg versus subcutaneous naratriptan 5 mg (Dahlof 1998).
Sumatriptan 6 mg versus subcutaneous naratriptan 10 mg (Dahlof 1998).
Sumatriptan 6 mg versus intravenous acetylsalicylic acid lysinate 1.8 g (Diener 1999).
Sumatriptan 6 mg versus subcutaneous alniditan 1.4 mg (Diener 2001).
Sumatriptan 6 mg versus subcutaneous alniditan 1.8 mg (Diener 2001).
Sumatriptan 6 mg versus intravenous LY293558 1.2 mg/kg (Sang 2004).
Sumatriptan 6 mg versus oral effervescent acetylsalicylic acid (ASA) 1000 mg + metoclopramide (MCP) 10 mg (S2BL99).
Sumatriptan 6 mg versus dihydroergotamine (DHE) nasal spray 1 mg (Touchon 1996).
Sumatriptan 6 mg versus subcutaneous DHE 1 mg (Winner 1996).
Sumatriptan 8 mg with placebo (Dahlof 1992; Mathew 1992; Ferrari 1991).
In total, 200 participants were treated with sumatriptan 1 mg, 201 with sumatriptan 2 mg, 202 with sumatriptan 3 mg, 442 with sumatriptan 4 mg, 4334 with sumatriptan 6 mg, 167 with sumatriptan 8 mg, 3018 with placebo, 60 with naratriptan 0.5 mg, 55 with naratriptan 1 mg, 42 with naratriptan 2.5 mg, 34 with naratriptan 5 mg, 34 with naratriptan 10 mg, 119 with intravenous acetylsalicylic acid lysinate 1.8 g, 309 with alniditan 1.4 mg, 141 with alniditan 1.8 mg, 13 with intravenous LY293558 1.2 mg/kg, 130 with oral effervescent acetylsalicylic acid (ASA) 1000 mg + metoclopramide (MCP) 10 mg, 277 with dihydroergotamine (DHE) nasal spray 1 mg, and 152 with subcutaneous DHE 1 mg.
Some studies were inconsistent in the treatment group denominators reported, so that the population varied slightly in size for different outcomes or at different time points. Where this variability was not explained in the text, the denominators were changed to match the treated efficacy population if this gave a more conservative estimate of the efficacy of the drug.
Full details of included studies are provided in the Characteristics of included studies table.
Excluded studies
We excluded 12 studies after reading the full report (Burke‐Ramirez 2001; Cady 1991; Cull 2001; Ensink 1991; Friedman 2005; Friedman 2006; Gonzalez‐Espinosa 1997; Melchart 2003; Pradel 2006; Russell 1995; S2BM04; Solbach 1993). The reasons for these exclusions are provided in the Characteristics of excluded studies table.
Risk of bias in included studies
Included studies were all randomised and double‐blind. The majority of the studies provided information about withdrawals and dropouts, although five studies either made no statement about withdrawals or did not give an adequate explantation for differing treatment group denominators. The reliability of the trials was determined using the Oxford Quality Scale. Six studies scored 5 of 5 on the scale, 10 studies scored 4 of 5, 17 studies scored 3 of 5, and two studies scored 2 of 5. Points were lost due to inadequate description of the methods of randomisation or double‐blinding, and also lack of information about withdrawals and dropouts. Details are provided in the Characteristics of included studies table.
In addition we created a 'Risk of bias' table which considered random sequence generation, allocation concealment, blinding, and study size (Figure 1). We considered no studies to be at high risk of bias from random sequence generation, allocation concealment, or blinding. Fifteen studies (Akpunonu 1995; Bates 1994; Bousser 1993; Cady 1993; Dahlof 1992; Dahlof 1998; Diener 1999; Gross 1994; Henry 1993; Mathew 1992; Mushet 1996 Study 1 and Study 2; Sang 2004; Schulman 2000; Thomson 1993) did not include 50 or more participants in each treatment arm and we therefore considered them to be at high risk of bias from their size.
1.
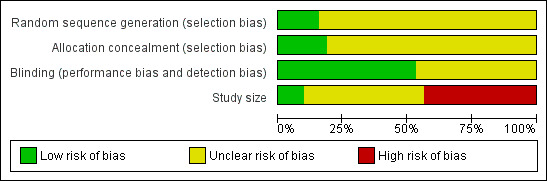
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Effects of interventions
Details of results for efficacy in individual studies are provided in Appendix 5.
Pain‐free at two hours
Sumatriptan 4 mg versus placebo
Two studies (664 participants) provided data (Mathew 1992; Wendt 2006).
The proportion of participants pain‐free at two hours with sumatriptan 4 mg was 49% (201/411; range 33% to 50%).
The proportion of participants pain‐free at two hours with placebo was 9% (23/253; range 3% to 11%).
The relative benefit of treatment compared with placebo was 4.8 (3.2 to 7.2; Analysis 1.1); the NNT was 2.5 (2.2 to 3.0).
1.1. Analysis.
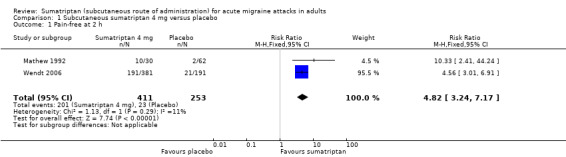
Comparison 1 Subcutaneous sumatriptan 4 mg versus placebo, Outcome 1 Pain‐free at 2 h.
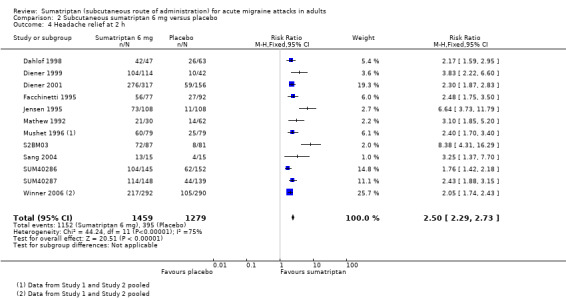
Sumatriptan 6 mg versus placebo
Thirteen studies (2522 participants) provided data (Dahlof 1998; Diener 1999; Diener 2001; Facchinetti 1995; Mathew 1992; Mushet 1996 Study 1 and Study 2; S2BM03; Sang 2004; SUM40286; SUM40287; Winner 2006 Study 1 and Study 2).
The proportion of participants pain‐free at two hours with sumatriptan 6 mg was 59% (799/1351; range 48% to 76%).
The proportion of participants pain‐free at two hours with placebo was 15% (174/1171; range 3% to 19%).
The relative benefit of treatment compared with placebo was 3.9 (3.3 to 4.5; Analysis 2.1; Figure 2); the NNT was 2.3 (2.1 to 2.4).
2.1. Analysis.
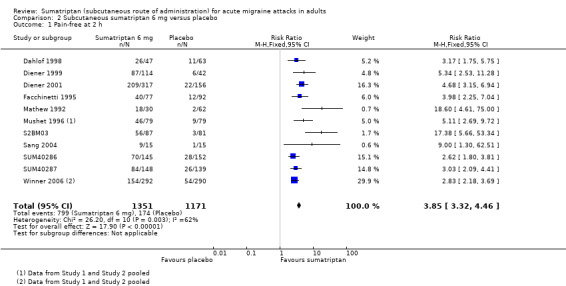
Comparison 2 Subcutaneous sumatriptan 6 mg versus placebo, Outcome 1 Pain‐free at 2 h.
2.
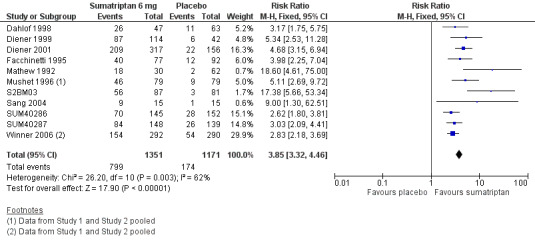
Forest plot of comparison: 2 Subcutaneous sumatriptan 6 mg versus placebo, outcome: 2.1 Pain‐free at 2 h.
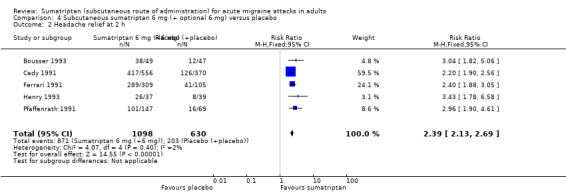
Sumatriptan 6 mg plus optional 6 mg versus placebo
Three studies (388 participants) provided data comparing sumatriptan 6 mg (with an optional second dose of sumatriptan 6 mg if initial relief was inadequate after one hour) with placebo (with an optional second dose of placebo if initial relief was inadequate) for a pain‐free response at two hours (Bousser 1993; Henry 1993; Pfaffenrath 1991). Overall, 34% (range 22% to 53%) of sumatriptan‐treated participants providing data for this comparison received two doses of medication (i.e. 6 mg + 6 mg), while 77% (range 74% to 81%) of placebo‐treated participants providing data received two doses of placebo.
The proportion of participants pain‐free at two hours with sumatriptan 6 mg (+ 6 mg) was 50% (117/233; range 47% to 51%).
The proportion of participants pain‐free at two hours with placebo (+ placebo) was 11% (17/155; range 8% to 15%).
The relative benefit of treatment compared with placebo was 4.6 (2.9 to 7.4; Analysis 4.1); the NNT was 2.6 (2.1 to 3.2).
4.1. Analysis.
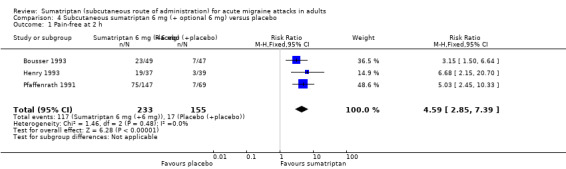
Comparison 4 Subcutaneous sumatriptan 6 mg (+ optional 6 mg) versus placebo, Outcome 1 Pain‐free at 2 h.
There was no significant difference in efficacy between a single dose of sumatriptan 6 mg and an initial dose of sumatriptan 6 mg plus an optional second dose after one hour in the event of inadequate relief from the initial dose.
Other doses of sumatriptan versus placebo
Two studies (Dahlof 1992; Mathew 1992) provided data comparing sumatriptan 8 mg with placebo, although the number of participants involved in this comparison was not sufficiently large to allow pooled analysis. Between 53% and 63% of participants treated with sumatriptan 8 mg were pain‐free at two hours compared with 0% to 3% of participants treating with placebo.
One study (Mathew 1992) provided data comparing sumatriptan 1 mg, 2 mg, and 3 mg with placebo, but there were insufficient data to carry out pooled analysis of these doses. The proportion of participants pain‐free at two hours after treatment with sumatriptan 1, 2, and 3 mg was 20%, 10%, and 27%, respectively, while only 3% of placebo‐treated participants were pain‐free at two hours.
Sumatriptan versus active comparators
Six studies (Dahlof 1998; Diener 1999; Diener 2001; S2BL99; Sang 2004; Touchon 1996) provided data comparing sumatriptan with an active comparator for pain‐free at two hours. None of these studies used comparable active comparators so no pooled analysis could be carried out.
Dahlof 1998 provided data comparing sumatriptan 6 mg with naratriptan at doses of 0.5, 1, 2.5, 5, and 10 mg. The proportion of participants pain‐free at two hours after treating with sumatriptan was 55%, compared to 30%, 44%, 60%, 79%, and 88% of participants treating with subcutaneous naratriptan 0.5, 1, 2.5, 5, and 10 mg, respectively.
Diener 1999 provided data comparing sumatriptan 6 mg with intravenous acetylsalicylic acid lysinate 1.8 g. The proportion of participants pain‐free at two hours after treating with sumatriptan was 76%, compared to 44% of participants treating with acetylsalicylic acid lysinate.
Diener 2001 provided data comparing sumatriptan 6 mg with subcutaneous alniditan 1.4 mg and 1.6 mg. The proportion of participants pain‐free at two hours after treating with sumatriptan was 66%, compared to 56% and 62% of participants treating with alniditan 1.4 mg and 1.6 mg, respectively.
S2BL99 provided data comparing sumatriptan 6 mg with oral effervescent ASA 1000 mg + MCP 10 mg. The proportion of participants pain‐free at two hours after treating with sumatriptan was 61%, compared to 37% of participants treating with oral ASA + MCP.
Sang 2004 provided data comparing sumatriptan 6 mg with intravenous LY293558 1.2 mg/kg. The proportion of participants pain‐free at two hours after treating with sumatriptan was 60%, compared to 54% of participants treating with LY293558.
Touchon 1996 provided data comparing sumatriptan 6 mg with DHE nasal spray 1 mg. The proportion of participants pain‐free at two hours after treating with sumatriptan was 66%, compared to 31% of participants treating with DHE nasal spray.
Pain‐free at one hour
Sumatriptan 4 mg versus placebo
Two studies (664 participants) provided data (Mathew 1992; Wendt 2006).
The proportion of participants pain‐free at one hour with sumatriptan 4 mg was 33% (134/411; range 17% to 34%).
The proportion of participants pain‐free at one hour with placebo was 6% (16/253; range 3% to 7%).
The relative benefit of treatment compared with placebo was 4.7 (2.8 to 7.7; Analysis 1.2); the NNT was 3.8 (3.2 to 4.8).
1.2. Analysis.
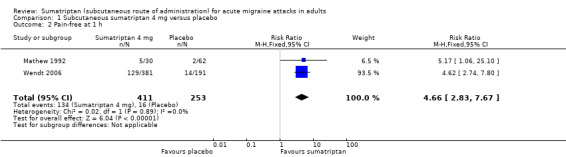
Comparison 1 Subcutaneous sumatriptan 4 mg versus placebo, Outcome 2 Pain‐free at 1 h.
Sumatriptan 6 mg versus placebo
Sixteen studies (3592 participants) provided data (Bousser 1993; Cady 1991 Study 1 and Study 2; Cady 1993; Facchinetti 1995; Ferrari 1991; Henry 1993; Jensen 1995; Mathew 1992; Mushet 1996 Study 1 and Study 2; Pfaffenrath 1991; S2BM03; Sang 2004; SUM40286; SUM40287).
The proportion of participants pain‐free at one hour with sumatriptan 6 mg was 41% (905/2198; range 27% to 49%).
The proportion of participants pain‐free at one hour with placebo was 7% (99/1394; range 1% to 11%).
The relative benefit of treatment compared with placebo was 5.6 (4.6 to 6.8; Analysis 2.2); the NNT was 2.9 (2.7 to 3.2).
2.2. Analysis.
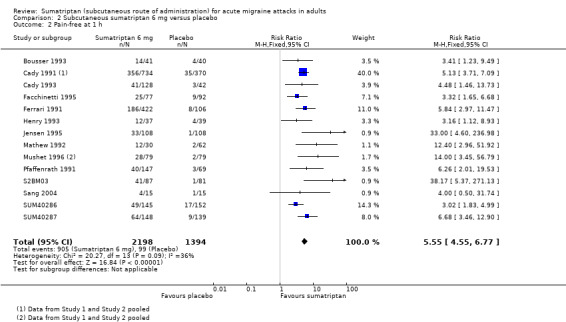
Comparison 2 Subcutaneous sumatriptan 6 mg versus placebo, Outcome 2 Pain‐free at 1 h.
Sumatriptan 6 mg was significantly more effective than sumatriptan 4 mg for complete relief of pain by one hour (z = 2.560; P = 0.011; see Summary of results B).
Sumatriptan 8 mg versus placebo
Two studies (308 participants) provided data (Ferrari 1991; Mathew 1992).
The proportion of participants pain‐free at one hour with sumatriptan 8 mg was 46% (65/140; range 33% to 50%).
The proportion of participants pain‐free at one hour with placebo was 6% (10/168; range 3% to 8%).
The relative benefit of treatment compared with placebo was 7.1 (3.8 to 13; Analysis 3.1); the NNT was 2.5 (2.0 to 3.2).
3.1. Analysis.
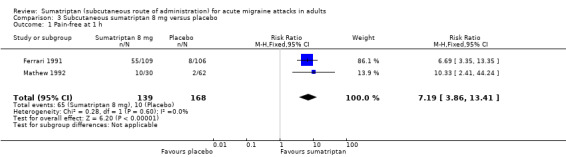
Comparison 3 Subcutaneous sumatriptan 8 mg versus placebo, Outcome 1 Pain‐free at 1 h.
Other doses of sumatriptan versus placebo
One study (Mathew 1992) provided data comparing sumatriptan 1 mg, 2 mg, and 3 mg with placebo, but there were insufficient data to carry out pooled analysis of these doses. The proportion of participants pain‐free at one hour after treatment with sumatriptan 1, 2, and 3 mg was 13%, 3%, and 23%, respectively, while only 3% of placebo‐treated participants were pain‐free at one hour.
Sumatriptan versus active comparators
Three studies (S2BL99; Sang 2004; Touchon 1996) provided data comparing sumatriptan with an active comparator for pain‐free at one hour. The two studies used different active comparators so no pooled analysis could be carried out.
S2BL99 provided data comparing sumatriptan 6 mg with oral effervescent ASA 1000 mg + MCP 10 mg. The proportion of participants pain‐free at one hour after treating with sumatriptan was 45%, compared to 21% of participants treating with oral ASA + MCP.
Sang 2004 provided data comparing sumatriptan 6 mg with intravenous LY293558 1.2 mg/kg. The proportion of participants pain‐free at one hour after treating with sumatriptan was 27%, compared to 31% of participants treating with LY293558.
Touchon 1996 provided data comparing sumatriptan 6 mg with DHE nasal spray 1 mg. The proportion of participants pain‐free at one hour after treating with sumatriptan was 47%, compared to 13% of participants treating with DHE nasal spray.
Headache relief at one hour
Sumatriptan 4 mg versus placebo
Two studies (664 participants) provided data (Mathew 1992; Wendt 2006).
The proportion of participants with headache relief at one hour with sumatriptan 4 mg was 66% (271/411; range 50% to 67%).
The proportion of participants with headache relief at one hour with placebo was 25% (64/253; range 24% to 26%).
The relative benefit of treatment compared with placebo was 2.6 (2.0 to 3.2; Analysis 1.3); the NNT was 2.5 (2.1 to 3.0).
1.3. Analysis.
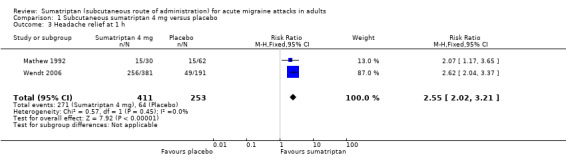
Comparison 1 Subcutaneous sumatriptan 4 mg versus placebo, Outcome 3 Headache relief at 1 h.
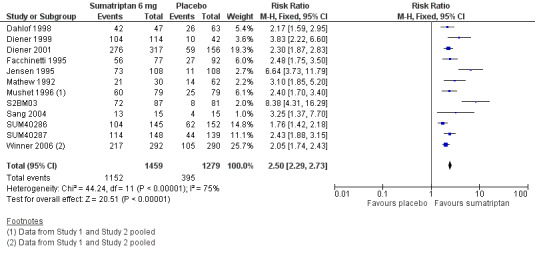
Sumatriptan 6 mg versus placebo
Twenty‐four studies (5177 participants) provided data (Bates 1994; Bousser 1993; Cady 1991 Study 1 and Study 2; Cady 1993; Dahlof 1998; Diener 1999; Diener 2001; Facchinetti 1995; Ferrari 1991; Gross 1994; Henry 1993; Jensen 1995; Mathew 1992; Mushet 1996 Study 1 and Study 2; Pfaffenrath 1991; S2BM03; Sang 2004; Schulman 2000; SUM40286; SUM40287; Winner 2006 Study 1 and Study 2).
The proportion of participants with headache relief at one hour with sumatriptan 6 mg was 71% (2229/3139; range 51% to 88%).
The proportion of participants with headache relief at one hour with placebo was 26% (532/2038; range 6% to 41%).
The relative benefit of treatment compared with placebo was 2.7 (2.5 to 2.9; Analysis 2.3; Figure 3); the NNT was 2.2 (2.1 to 2.4).
2.3. Analysis.
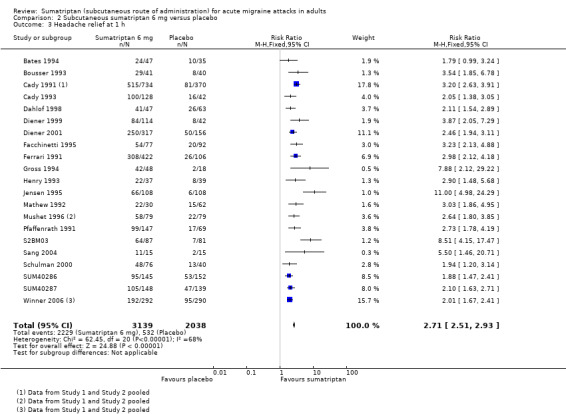
Comparison 2 Subcutaneous sumatriptan 6 mg versus placebo, Outcome 3 Headache relief at 1 h.
3.
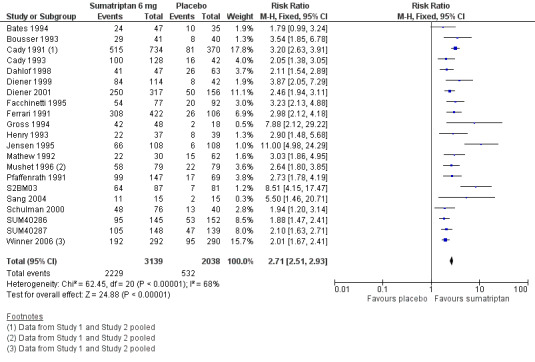
Forest plot of comparison: 2 Subcutaneous sumatriptan 6 mg versus placebo, outcome: 2.3 Headache relief at 1 h.
Sumatriptan 8 mg versus placebo
Three studies (361 participants) provided data (Dahlof 1992; Ferrari 1991; Mathew 1992).
The proportion of participants with headache relief at one hour with sumatriptan 8 mg was 80% (133/166; range 79% to 85%).
The proportion of participants with headache relief at one hour with placebo was 23% (44/195; range 11% to 25%).
The relative benefit of treatment compared with placebo was 3.6 (2.7 to 4.7; Analysis 3.2); the NNT was 1.7 (1.5 to 2.0).
3.2. Analysis.
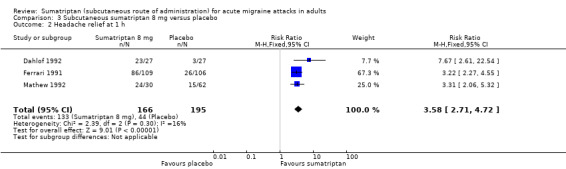
Comparison 3 Subcutaneous sumatriptan 8 mg versus placebo, Outcome 2 Headache relief at 1 h.
Sumatriptan 8 mg was significantly more effective than sumatriptan 6 mg for headache relief at one hour (z = 2.818; P = 0.005; see Summary of results B).
Other doses of sumatriptan versus placebo
One study (Mathew 1992) provided data comparing sumatriptan 1 mg, 2 mg, and 3 mg with placebo, but there were insufficient data to carry out pooled analysis of these doses. The proportion of participants with headache relief at one hour after treatment with sumatriptan 1, 2, and 3 mg was 43%, 57%, and 57%, respectively, while only 24% of placebo‐treated participants had relief at one hour.
Sumatriptan versus active comparators
Seven studies (Dahlof 1998; Diener 1999; Diener 2001; S2BL99; Sang 2004; Touchon 1996; Winner 1996) provided data comparing sumatriptan with an active comparator for headache relief at one hour. None of these studies used comparable active comparators so no pooled analysis could be carried out.
Dahlof 1998 provided data comparing sumatriptan 6 mg with subcutaneous naratriptan at doses of 0.5, 1, 2.5, 5, and 10 mg. The proportion of participants with headache relief at one hour after treating with sumatriptan was 87%, compared to 60%, 64%, 81%, 85%, and 76% of participants treating with naratriptan 0.5, 1, 2.5, 5, and 10 mg, respectively.
Diener 1999 provided data comparing sumatriptan 6 mg with intravenous acetylsalicylic acid lysinate 1.8 g. The proportion of participants with headache relief at one hour after treating with sumatriptan was 74%, compared to 60% of participants treating with acetylsalicylic acid lysinate.
Diener 2001 provided data comparing sumatriptan 6 mg with subcutaneous alniditan 1.4 mg and 1.6 mg. The proportion of participants with headache relief at one hour after treating with sumatriptan was 79%, compared to 75% and 81% of participants treating with alniditan 1.4 mg and 1.6 mg, respectively.
S2BL99 provided data comparing sumatriptan 6 mg with oral effervescent ASA 1000 mg + MCP 10 mg. The proportion of participants with headache relief at one hour after treating with sumatriptan was 71%, compared with 46% of participants treating with oral ASA + MCP.
Sang 2004 provided data comparing sumatriptan 6 mg with intravenous LY293558 1.2 mg/kg. The proportion of participants with headache relief at one hour after treating with sumatriptan was 73%, compared to 69% of participants treating with LY293558.
Touchon 1996 provided data comparing sumatriptan 6 mg with DHE nasal spray 1 mg. The proportion of participants with headache relief at one hour after treating with sumatriptan was 71%, compared to 34% of participants treating with DHE nasal spray.
Winner 1996 provided data comparing sumatriptan 6 mg with subcutaneous DHE 1 mg. The proportion of participants with headache relief at one hour after treating with sumatriptan was 78%, compared to 57% of participants treating with DHE.
Headache relief at two hours
Sumatriptan 4 mg versus placebo
Two studies (664 participants) provided data (Mathew 1992; Wendt 2006).
The proportion of participants with headache relief at two hours with sumatriptan 4 mg was 70% (286/411; range 60% to 70%).
The proportion of participants with headache relief at two hours with placebo was 22% (56/253; range 22% to 23%).
The relative benefit of treatment compared with placebo was 3.1 (2.4 to 4.0; Analysis 1.4); the NNT was 2.1 (1.8 to 2.5).
1.4. Analysis.
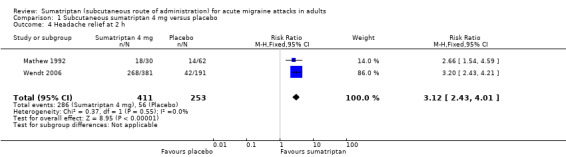
Comparison 1 Subcutaneous sumatriptan 4 mg versus placebo, Outcome 4 Headache relief at 2 h.
Sumatriptan 6 mg versus placebo
Fourteen studies (2738 participants) provided data (Dahlof 1998; Diener 1999; Diener 2001; Facchinetti 1995; Jensen 1995; Mathew 1992; Mushet 1996 Study 1 and Study 2; S2BM03; Sang 2004; SUM40286; SUM40287; Winner 2006 Study 1 and Study 2).
The proportion of participants with headache relief at two hours with sumatriptan 6 mg was 79% (1152/1459; range 68% to 91%).
The proportion of participants with headache relief at two hours with placebo was 31% (395/1279; range 10% to 41%).
The relative benefit of treatment compared with placebo was 2.5 (2.3 to 2.7; Analysis 2.4; Figure 4); the NNT was 2.1 (2.0 to 2.2).
2.4. Analysis.
Comparison 2 Subcutaneous sumatriptan 6 mg versus placebo, Outcome 4 Headache relief at 2 h.
4.
Forest plot of comparison: 2 Subcutaneous sumatriptan 6 mg versus placebo, outcome: 2.4 Headache relief at 2 h.
Sumatriptan 6 mg plus optional 6 mg versus placebo
Six studies (1728 participants) provided data comparing sumatriptan 6 mg (with an optional second dose of sumatriptan 6 mg if initial relief was inadequate after one hour) with placebo (with an optional second dose of placebo if initial relief was inadequate) for headache relief at two hours (Bousser 1993; Cady 1991 Study 1 and Study 2; Ferrari 1991; Henry 1993; Pfaffenrath 1991). Overall, 30% (range 22% to 53%) of sumatriptan‐treated participants providing data for this comparison received two doses of medication (i.e. 6 mg + 6 mg), while 87% (range 74% to 91%) of placebo‐treated participants providing data received two doses of placebo.
The proportion of participants with headache relief at two hours with sumatriptan 6 mg (+ 6 mg) was 79% (871/1098; range 69% to 94%).
The proportion of participants with headache relief at two hours with placebo (+ placebo) was 32% (203/630; range 21% to 39%).
The relative benefit of treatment compared with placebo was 2.4 (2.1 to 2.7; Analysis 4.2); the NNT was (1.9 to 2.3).
4.2. Analysis.
Comparison 4 Subcutaneous sumatriptan 6 mg (+ optional 6 mg) versus placebo, Outcome 2 Headache relief at 2 h.
There was no significant difference in efficacy between a single dose of sumatriptan 6 mg and an initial dose of sumatriptan 6 mg plus an optional second dose in the event of inadequate relief after one hour from the initial dose.
Other doses of sumatriptan versus placebo
Two studies (Dahlof 1992; Mathew 1992) provided data comparing sumatriptan 8 mg with placebo, although the number of participants involved in this comparison was not sufficiently large to allow pooled analysis. Between 85% and 87% of participants treated with sumatriptan 8 mg had headache relief at two hours compared with 23% of participants treating with placebo.
One study (Mathew 1992) provided data comparing sumatriptan 1 mg, 2 mg, and 3 mg with placebo, but there were insufficient data to carry out pooled analysis of these doses. The proportion of participants with headache relief at two hours after treatment with sumatriptan 1, 2, and 3 mg was 40%, 47%, and 57%, respectively, while only 23% of placebo‐treated participants had relief at two hours.
Sumatriptan versus active comparators
Seven studies (Dahlof 1998; Diener 1999; Diener 2001; S2BL99; Sang 2004; Touchon 1996; Winner 1996) provided data comparing sumatriptan with an active comparator for headache relief at two hours. None of these studies used comparable active comparators so no pooled analysis could be carried out.
Dahlof 1998 provided data comparing sumatriptan 6 mg with subcutaneous naratriptan at doses of 0.5, 1, 2.5, 5, and 10 mg. The proportion of participants with headache relief at two hours after treating with sumatriptan was 89%, compared to 65%, 75%, 83%, 94%, and 91% of participants treating with naratriptan 0.5, 1, 2.5, 5, and 10 mg, respectively.
Diener 1999 provided data comparing sumatriptan 6 mg with intravenous acetylsalicylic acid lysinate 1.8 g. The proportion of participants with headache relief at two hours after treating with sumatriptan was 91%, compared to 74% of participants treating with acetylsalicylic acid lysinate.
Diener 2001 provided data comparing sumatriptan 6 mg with subcutaneous alniditan 1.4 mg and 1.6 mg. The proportion of participants with headache relief at two hours after treating with sumatriptan was 87%, compared to 81% and 85% of participants treating with alniditan 1.4 mg and 1.6 mg, respectively.
S2BL99 provided data comparing sumatriptan 6 mg with oral effervescent ASA 1000 mg + MCP 10 mg. The proportion of participants with headache relief at two hours after treating with sumatriptan was 81%, compared to 63% of participants treated with oral ASA + MCP.
Sang 2004 provided data comparing sumatriptan 6 mg with intravenous LY293558 1.2 mg/kg. The proportion of participants with headache relief at two hours after treating with sumatriptan was 87%, compared to 69% of participants treating with LY293558.
Touchon 1996 provided data comparing sumatriptan 6 mg with DHE nasal spray 1 mg. The proportion of participants with headache relief at two hours after treating with sumatriptan was 81%, compared to 52% of participants treating with DHE nasal spray.
Winner 1996 provided data comparing sumatriptan 6 mg with subcutaneous DHE 1 mg. The proportion of participants with headache relief at one hour after treating with sumatriptan was 85%, compared to 73% of participants treating with DHE.
Sustained pain‐free during the 24 hours postdose
Sumatriptan 6 mg versus placebo
Five studies (1336 participants) provided data (Cady 1993; SUM40286; SUM40287; Winner 2006 Study 1 and Study 2).
The proportion of participants with a 24‐hour sustained pain‐free response with sumatriptan 6 mg was 31% (222/713; range 20% to 34%).
The proportion of participants with a 24‐hour sustained pain‐free response with placebo was 15% (91/623; range 12% to 15%).
The relative benefit of treatment compared with placebo was 2.2 (1.8 to 2.8; Analysis 2.5); the NNT was 6.1 (4.8 to 8.2).
2.5. Analysis.
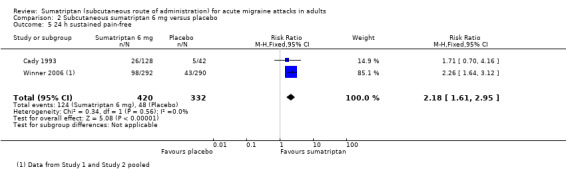
Comparison 2 Subcutaneous sumatriptan 6 mg versus placebo, Outcome 5 24 h sustained pain‐free.
| Summary of results A: Pain‐free and headache relief in placebo controlled studies | ||||||
| Studies |
Attacks treated |
Treatment (%) |
Placebo (%) |
Relative risk (95% CI) |
NNT (95% CI) |
|
| Pain‐free at 2 hours | ||||||
| Sumatriptan 4 mg | 2 | 664 | 49 | 9 | 4.8 (3.2 to 7.2) | 2.5 (2.2 to 3.0) |
| Sumatriptan 6 mg | 13 | 2522 | 59 | 15 | 3.9 (3.3 to 4.5) | 2.3 (2.1 to 2.4) |
| Sumatriptan 6 mg (+ 6 mg) | 3 | 388 | 50 | 11 | 4.6 (2.9 to 7.4) | 2.6 (2.1 to 3.2) |
| Pain‐free at 1 hour | ||||||
| Sumatriptan 4 mg | 2 | 664 | 33 | 6 | 4.7 (2.8 to 7.7) | 3.8 (3.2 to 4.8) |
| Sumatriptan 6 mg | 16 | 3592 | 41 | 7 | 5.6 (4.6 to 6.8) | 2.9 (2.7 to 3.2) |
| Sumatriptan 8 mg | 2 | 308 | 46 | 6 | 7.1 (3.8 to 13) | 2.5 (2.0 to 3.2) |
| Headache relief at 1 hour | ||||||
| Sumatriptan 4 mg | 2 | 664 | 66 | 25 | 2.6 (2.0 to 3.2) | 2.5 (2.1 to 3.0) |
| Sumatriptan 6 mg | 24 | 5177 | 71 | 26 | 2.7 (2.5 to 2.9) | 2.2 (2.1 to 2.4) |
| Sumatriptan 8 mg | 3 | 361 | 80 | 23 | 3.6 (2.7 to 4.7) | 1.7 (1.5 to 2.0) |
| Headache relief at 2 hours | ||||||
| Sumatriptan 4 mg | 2 | 664 | 70 | 22 | 3.1 (2.4 to 4.0) | 2.1 (1.8 to 2.5) |
| Sumatriptan 6 mg | 14 | 2738 | 79 | 31 | 2.5 (2.3 to 2.7) | 2.1 (2.0 to 2.2) |
| Sumatriptan 6 mg (+6 mg) | 6 | 1728 | 79 | 32 | 2.4 (2.1 to 2.7) | 2.1 (1.9 to 2.3) |
| Sustained pain‐free during the 24 hours postdose | ||||||
| Sumatriptan 6 mg | 5 | 1336 | 31 | 15 | 2.2 (1.8 to 2.8) | 6.1 (4.8 to 8.2) |
| Summary of results B: Statistical tests for the effect of dose | ||
| z | P | |
| Pain‐free at 1 hour | ||
| Sumatriptan 4 mg versus sumatriptan 6 mg | 2.560 | 0.011 |
| Headache relief at 1 hour | ||
| Sumatriptan 6 mg versus sumatriptan 8 mg | 2.818 | 0.005 |
Sensitivity analyses
A summary of all sensitivity analyses carried out is available in Appendix 6.
Methodological quality
We carried out sensitivity analyses to take into consideration and assess the effect of variation in methodological quality of the included studies. We considered studies with an Oxford Quality Score of 2 of 5 to be at greater risk of bias and therefore analysed these separately for each outcome. Where there were insufficient data to provide a meaningful comparison of these lower‐quality trials with the higher‐quality trials (scoring 3 or more of 5) for a particular outcome, we performed sensitivity analyses simply to remove the lower‐quality trials from the original all‐trials analyses.
Only one study (Mathew 1992) considered to be of low methodological quality provided data for pooled efficacy analyses. Removing this study from pooled analyses of efficacy for the 4 mg dose would have made any further analyses meaningless (leaving only one study to provide data) and therefore was not done. Removing this study from the analyses of pain‐free at one and two hours, as well as headache relief at one and two hours for sumatriptan 6 mg, made no significant difference to the calculated relative benefit of treatment versus placebo (analyses not shown).
Size of treatment arms
Due to the large number of studies that did not include 50 or more participants in each treatment arm (which were therefore considered to be at high risk of bias from their size), we performed sensitivity analyses to investigate the potential effect of study size on estimates of treatment efficacy. Only the 6 mg dose of sumatriptan provided enough data to carry out these sensitivity analyses.
Pain‐free at two hours
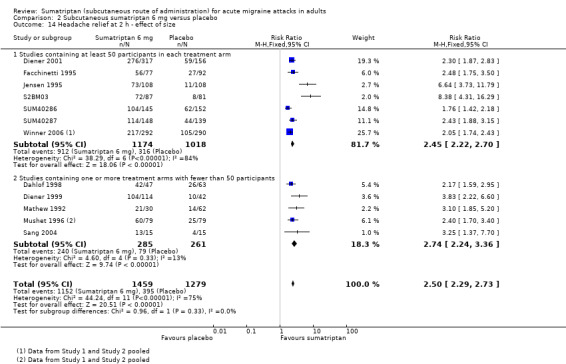
Of the 13 studies originally analysed comparing sumatriptan 6 mg with placebo, seven had at least 50 participants in each treatment arm (Diener 2001; Facchinetti 1995; S2BM03; SUM40286; SUM40287; Winner 2006 Study 1 and Study 2). When these and the remaining studies (where one or more treatment arms contained fewer than 50 participants) were analysed separately, a significant difference in treatment effect was observed (z = 3.195, P = 0.001; Analysis 2.11).
2.11. Analysis.
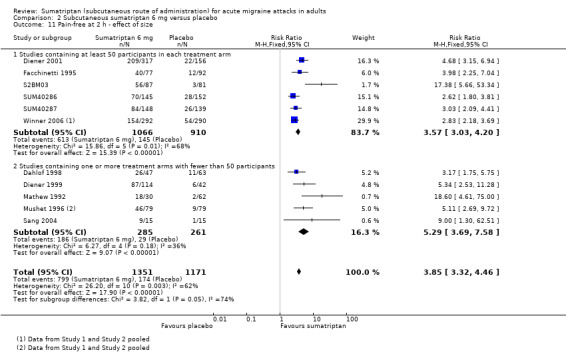
Comparison 2 Subcutaneous sumatriptan 6 mg versus placebo, Outcome 11 Pain‐free at 2 h ‐ effect of size.
For studies with at least 50 participants in each treatment arm, the relative benefit of treatment compared with placebo was 3.6 (3.0 to 4.2); the NNT was 2.4 (2.2 to 2.7).
For studies with at least one treatment arm containing fewer than 50 participants, the relative benefit of treatment compared with placebo was 5.3 (3.7 to 7.6); the NNT was 1.9 (1.6 to 2.1).
Pain‐free at one hour
Of the 16 studies originally analysed comparing sumatriptan 6 mg with placebo, nine had at least 50 participants in each treatment arm (Cady 1991 Study 1 and Study 2; Facchinetti 1995; Ferrari 1991; Jensen 1995; Pfaffenrath 1991; S2BM03; SUM40286; SUM40287). When these and the remaining studies (where one or more treatment arms contained fewer than 50 participants) were analysed separately, a significant difference in treatment effect was observed (z = 2.210, P = 0.027; Analysis 2.12).
2.12. Analysis.
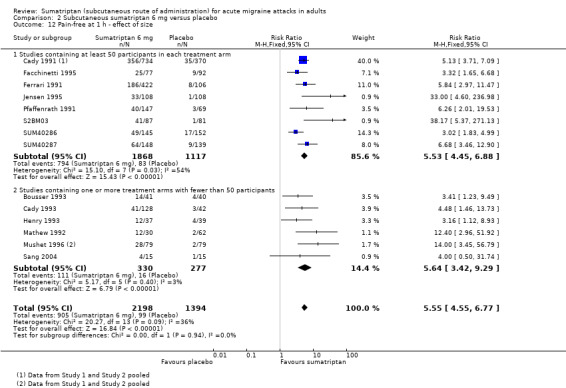
Comparison 2 Subcutaneous sumatriptan 6 mg versus placebo, Outcome 12 Pain‐free at 1 h ‐ effect of size.
For studies with at least 50 participants in each treatment arm, the relative benefit of treatment compared with placebo was 5.5 (4.5 to 6.9); the NNT was 2.9 (2.7 to 3.1).
For studies with at least one treatment arm containing fewer than 50 participants, the relative benefit of treatment compared with placebo was 5.6 (3.4 to 9.3); the NNT was 3.6 (3.0 to 4.5).
Headache relief at one hour
Of the 24 studies originally analysed comparing sumatriptan 6 mg with placebo, 12 had at least 50 participants in each treatment arm (Cady 1991 Study 1 and Study 2; Diener 2001; Facchinetti 1995; Ferrari 1991; Jensen 1995; Pfaffenrath 1991; S2BM03; SUM40286; SUM40287; Winner 2006 Study 1 and Study 2). When these and the remaining studies (where one or more treatment arms contained fewer than 50 participants) were analysed separately, there was no significant difference between the two groups (z = 0.145, P = 0.881; Analysis 2.13).
2.13. Analysis.
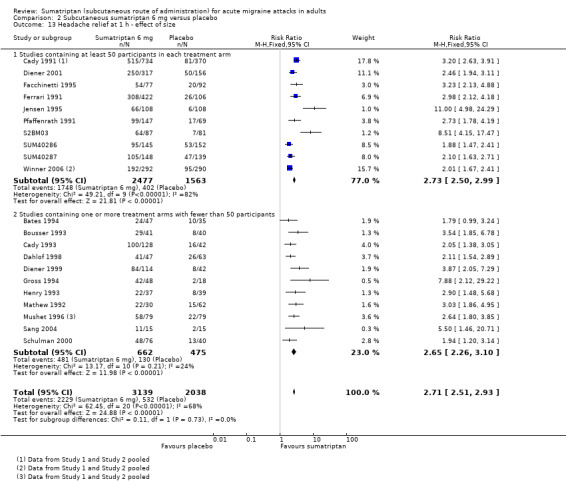
Comparison 2 Subcutaneous sumatriptan 6 mg versus placebo, Outcome 13 Headache relief at 1 h ‐ effect of size.
Headache relief at two hours
Of the 14 studies originally analysed comparing sumatriptan 6 mg with placebo, eight had at least 50 participants in each treatment arm (Diener 2001; Facchinetti 1995; Jensen 1995; S2BM03; SUM40286; SUM40287; Winner 2006 Study 1 and Study 2). When these and the remaining studies (where one or more treatment arms contained fewer than 50 participants) were analysed separately, there was no significant difference between the two groups (z = 1.806, P = 0.070; Analysis 2.14).
2.14. Analysis.
Comparison 2 Subcutaneous sumatriptan 6 mg versus placebo, Outcome 14 Headache relief at 2 h ‐ effect of size.
Missing data
Two studies (Jensen 1995; S2BM03) providing data for primary efficacy analyses reported only the results of participants completing both phases of a cross‐over design study; meaning that data for between 9% and 15% of participants were missing. We performed sensitivity analyses to investigate the potential effect of this missing data on estimates of treatment efficacy.
Pain‐free at one hour
Of the 16 studies originally analysed comparing sumatriptan 6 mg with placebo, two had substantial missing data (Jensen 1995; S2BM03). When these and the remaining studies (where there was no missing data) were analysed separately, there was no significant difference between the two groups (z = 0.908, P = 0.363; Analysis 2.15).
2.15. Analysis.
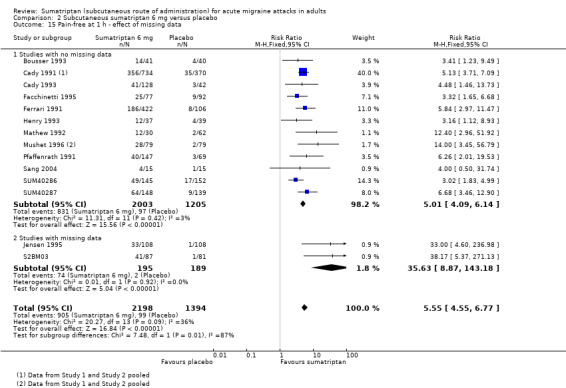
Comparison 2 Subcutaneous sumatriptan 6 mg versus placebo, Outcome 15 Pain‐free at 1 h ‐ effect of missing data.
Headache relief at one hour
Of the 24 studies originally analysed comparing sumatriptan 6 mg with placebo, two had substantial missing data (Jensen 1995; S2BM03). When these and the remaining studies (where there was no missing data) were analysed separately, a significant difference in treatment effect was observed (z = 4.068, P < 0.00006; Analysis 2.16).
2.16. Analysis.
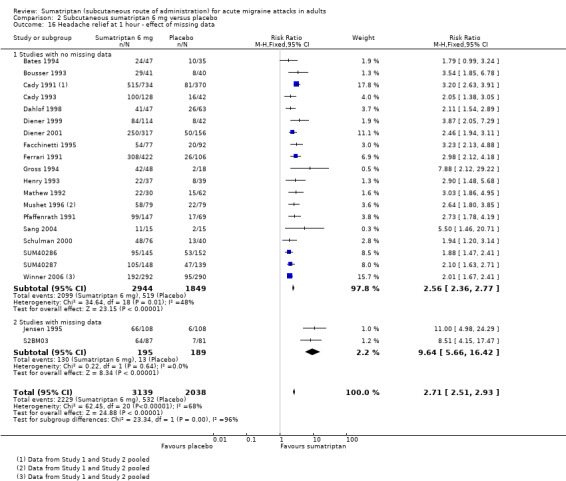
Comparison 2 Subcutaneous sumatriptan 6 mg versus placebo, Outcome 16 Headache relief at 1 hour ‐ effect of missing data.
For studies with no missing data, the relative benefit of treatment compared with placebo was 2.6 (2.4 to 2.8); the NNT was 2.3 (2.2 to 2.5).
For studies with substantial missing data, the relative benefit of treatment compared with placebo was 9.6 (5.7 to 16); the NNT was 1.7 (1.5 to 1.9).
Headache relief at two hours
Of the 14 studies originally analysed comparing sumatriptan 6 mg with placebo, two had substantial missing data (Jensen 1995; S2BM03). When these and the remaining studies (where there were no missing data) were analysed separately, a significant difference in treatment effect was observed (z = 4.520, P < 0.00006; Analysis 2.17).
2.17. Analysis.
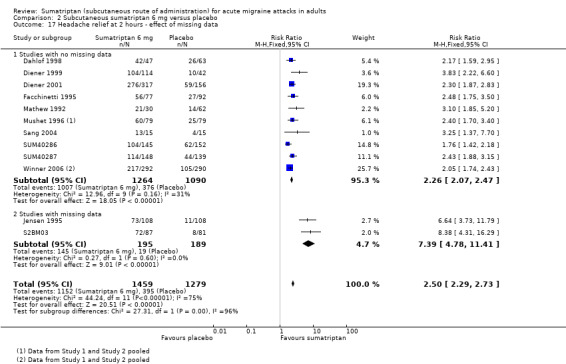
Comparison 2 Subcutaneous sumatriptan 6 mg versus placebo, Outcome 17 Headache relief at 2 hours ‐ effect of missing data.
For studies with no missing data, the relative benefit of treatment compared with placebo was 2.3 (2.1 to 2.5); the NNT was 2.2 (2.1 to 2.4).
For studies with substantial missing data, the relative benefit of treatment compared with placebo was 7.4 (4.8 to 11); the NNT was 1.6 (1.4 to 1.8).
Presence of aura
There were insufficient data to carry out any sensitivity analyses for participants with and without aura.
Use of rescue medication
All studies allowed participants whose symptoms were not adequately controlled to take additional rescue or 'escape' medication (usually a different analgesic, or in some studies a second dose of test medication). Participants were asked to wait, usually for two hours, before taking any additional medication in order to give the test medication enough time to have an effect. Use of rescue medication at or after a defined time point was reported in most studies and is a measure of treatment failure (lack of efficacy). The time over which use of rescue medication was measured varied between studies. Some reported use of rescue medication up to two hours after initial dosing, while the others reported use of rescue medication up to 24 hours after initial dosing.
Four studies reported data comparing sumatriptan with an active comparator for the use of rescue medication, but no quantitative analysis of these data was possible.
Sumatriptan 6 mg versus placebo
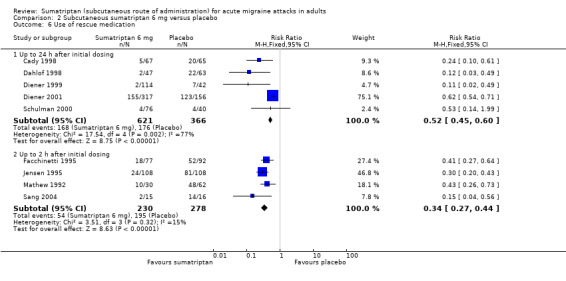
Five studies (987 participants) provided data for the use of rescue medication up to 24 hours after initial dosing (Cady 1998; Dahlof 1998; Diener 1999; Diener 2001; Schulman 2000).
The proportion of participants requiring rescue medication with sumatriptan 6 mg was 27% (168/621; range 2% to 49%).
The proportion of participants requiring rescue medication with placebo was 48% (176/366; range 10% to 79%).
The relative benefit of treatment compared with placebo was 0.52 (0.45 to 0.60; Analysis 2.6); the NNTp was 4.8 (3.7 to 6.7).
2.6. Analysis.
Comparison 2 Subcutaneous sumatriptan 6 mg versus placebo, Outcome 6 Use of rescue medication.
Four studies (508 participants) provided data for the use of rescue medication up to two hours after initial dosing (Facchinetti 1995; Jensen 1995; Mathew 1992; Sang 2004).
The proportion of participants requiring rescue medication with sumatriptan 6 mg was 23% (54/230; range 13% to 33%).
The proportion of participants requiring rescue medication with placebo was 70% (195/278; range 57% to 88%).
The relative benefit of treatment compared with placebo was 0.34 (0.27 to 0.43; Analysis 2.6); the NNTp was 2.1 (1.8 to 2.6).
Sumatriptan versus active comparators
Four studies (Dahlof 1998; Diener 1999; Diener 2001; S2BL99) provided data comparing sumatriptan with an active comparator for the use of rescue medication up to 24 hours after initial dosing. None of these studies used comparable active comparators so no pooled analysis could be carried out.
Dahlof 1998 provided data comparing sumatriptan 6 mg with naratriptan at doses of 0.5, 1, 2.5, 5, and 10 mg. The proportion of participants requiring rescue medication within 24 hours of treating with sumatriptan was 4%, compared to 35%, 22%, 12%, 6%, and 3% of participants treating with naratriptan 0.5, 1, 2.5, 5, and 10 mg, respectively.
Diener 1999 provided data comparing sumatriptan 6 mg with intravenous acetylsalicylic acid lysinate 1.8 g. The proportion of participants requiring rescue medication within 24 hours of treating with sumatriptan was 2%, compared to 4% of participants treating with acetylsalicylic acid lysinate.
Diener 2001 provided data comparing sumatriptan 6 mg with subcutaneous alniditan 1.4 mg and 1.6 mg. The proportion of participants requiring rescue medication within 24 hours of treating with sumatriptan was 49%, compared to 46% and 46% of participants treating with alniditan 1.4 mg and 1.6 mg, respectively.
S2BL99 provided data comparing sumatriptan 6 mg with oral effervescent ASA 1000 mg + MCP 10 mg. The proportion of participants requiring rescue medication within 24 hours of treating with sumatriptan was 22%, compared with 35% of participants treating with oral ASA + MCP.
Relief of headache‐associated symptoms
In general, relief of headache‐associated symptoms (defined as a symptom reduction from any intensity at baseline to none by a defined time point) was inconsistently reported. Of the 14 studies that reported any data for symptom relief at any time after administration of study medication, only five reported on relief of all four major symptoms of interest, and eight of the studies reported relief at one hour rather than the two hours we have analysed in the other reviews in this series. In addition, not all studies reported baseline incidence of associated symptoms from which relief could be calculated, although some did report presence of symptoms two hours after treatment. The incidence of vomiting was very low in all studies and where reported did not permit analysis.
Five of the studies providing data on relief of associated symptoms (Cady 1993; Facchinetti 1995; Pfaffenrath 1991; Wendt 2006; Winner 2006 Study 1) included a small number (< 10%) of participants with mild baseline pain intensity. It is possible that these participants had fewer or less severe associated symptoms, but the number was considered small enough that even if this were so, there would not be a major effect on the overall result; we therefore included these studies in any pooled analyses to which they were relevant.
There were only sufficient data to carry out pooled analyses of relief of associated symptoms for the 6 mg dose of sumatriptan.
Relief of nausea
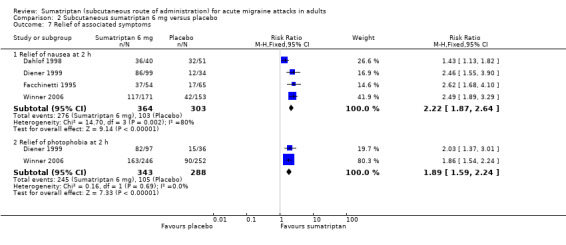
Five studies (667 participants) provided data comparing sumatriptan 6 mg with placebo for the relief of nausea at two hours after initial dosing (Dahlof 1998; Diener 1999; Facchinetti 1995; Winner 2006 Study 1 and Study 2).
The proportion of participants with relief of nausea at two hours with sumatriptan 6 mg was 76% (276/364; range 68% to 90%).
The proportion of participants with relief of nausea at two hours with placebo was 34% (103/303; range 26% to 63%).
The relative benefit of treatment compared with placebo was 2.2 (1.9 to 2.6; Analysis 2.7); the NNT was 2.4 (2.1 to 2.9).
2.7. Analysis.
Comparison 2 Subcutaneous sumatriptan 6 mg versus placebo, Outcome 7 Relief of associated symptoms.
Data were also provided by eight studies (1461 participants) comparing sumatriptan 6 mg with placebo for the relief of nausea at one hour after initial dosing (Cady 1991 Study 1 and Study 2; Cady 1993; Henry 1993; Mathew 1992; Mushet 1996 Study 1 and Study 2; Pfaffenrath 1991).
The relative benefit of treatment compared with placebo was 1.9 (1.7 to 2.2; analysis not shown); the NNT was 3.1 (2.7 to 3.7).
Two studies provided data comparing sumatriptan with an active comparator for the relief of nausea after treatment. Touchon 1996 reported 76% of participants treated with sumatriptan experiencing relief of nausea by two hours, compared with 54% of participants treated with DHE nasal spray 1 mg. Winner 1996 reported that 71% of sumatriptan‐treated participants had relief of nausea by one hour, compared with 50% of participants treated with subcutaneous DHE 1 mg. There were insufficient data for any pooled analyses.
Relief of photophobia
Three studies (631 participants) provided data comparing sumatriptan 6 mg with placebo for the relief of photophobia at two hours after initial dosing (Diener 1999; Winner 2006 Study 1 and Study 2).
The proportion of participants with relief of photophobia at two hours with sumatriptan 6 mg was 71% (245/343; range 66% to 85%).
The proportion of participants with relief of photophobia at two hours with placebo was 36% (105/288; range 36% to 42%).
The relative benefit of treatment compared with placebo was 1.9 (1.6 to 2.2; Analysis 2.7); the NNT was 2.9 (2.4 to 3.6).
Data were also provided by six studies (1460 participants) comparing sumatriptan 6 mg with placebo for the relief of photophobia at one hour after initial dosing (Cady 1991 Study 1 and Study 2; Cady 1993; Mathew 1992; Mushet 1996 Study 1 and Study 2).
The relative benefit of treatment compared with placebo was 3.0 (2.5 to 3.7; analysis not shown); the NNT was 2.7 (2.4 to 3.1).
Relief of phonophobia
Three studies (572 participants) provided data comparing sumatriptan 6 mg with placebo for the relief of phonophobia at two hours after initial dosing (Diener 1999; Winner 2006 Study 1 and Study 2).
The proportion of participants with relief of phonophobia at two hours with sumatriptan 6 mg was 72% (223/310; range 69% to 80%).
The proportion of participants with relief of phonophobia at two hours with placebo was 39% (101/262; range 38% to 41%).
The relative benefit of treatment compared with placebo was 1.8 (1.5 to 2.2) (Analysis 2.7); the NNT was 3.0 (2.4 to 3.9).
Data were also provided by three studies (300 participants) comparing sumatriptan 6 mg with placebo for the relief of phonophobia at one hour after dosing (Cady 1993; Mushet 1996 Study 1 and Study 2).
The relative benefit of treatment compared with placebo was 2.6 (1.8 to 3.7; analysis not shown); the NNT was 2.4 (1.9 to 3.3).
There were no significant differences between relief at one hour and relief at two hours for any of the analysed associated symptoms.
Sumatriptan versus active comparators
Four studies (Dahlof 1998; Diener 1999; S2BL99; Touchon 1996) provided data comparing sumatriptan with an active comparator for relief of nausea at two hours. None of these studies used comparable active comparators so no pooled analysis could be carried out.
Dahlof 1998 provided data comparing sumatriptan 6 mg with subcutaneous naratriptan at doses of 0.5, 1, 2.5, 5, and 10 mg. The proportion of participants with relief of nausea at two hours after treating with sumatriptan was 90%, compared to 74%, 92%, 91%, 96%, and 96% of participants treating with subcutaneous naratriptan 0.5, 1, 2.5, 5, and 10 mg, respectively.
Diener 1999 provided data comparing sumatriptan 6 mg with intravenous acetylsalicylic acid lysinate 1.8 g. The proportion of participants with relief of nausea at two hours after treating with sumatriptan was 87%, compared to 65% of participants treating with acetylsalicylic acid lysinate.
S2BL99 provided data comparing sumatriptan 6 mg with oral effervescent ASA 1000 mg + MCP 10 mg. The proportion of participants with relief of nausea at two hours after treating with sumatriptan was 77%, compared to 70% of participants treating with oral ASA + MCP.
Touchon 1996 provided data comparing sumatriptan 6 mg with DHE nasal spray 1 mg. The proportion of participants with relief of nausea at two hours after treating with sumatriptan was 76%, compared to 54% of participants treating with DHE nasal spray.
Only one study (Diener 1999) provided data comparing sumatriptan with an active comparator for the relief of photophobia and phonophobia at two hours. The proportion of participants with relief of photophobia at two hours after treating with sumatriptan 6 mg was 85%, compared to 77% of participants treating with intravenous acetylsalicylic acid lysinate 1.8 g. The proportion of participants with relief of phonophobia at two hours after treating with sumatriptan 6 mg was 80%, compared to 77% of participants treating with acetylsalicylic acid lysinate 1.8 g.
Presence of associated symptoms after two hours
We also analysed studies according to the presence of associated symptoms two hours after treatment, irrespective of whether they were present at baseline, and calculated NNTps (Appendix 7). Sumatriptan 6 mg significantly reduced the number of participants with nausea, photophobia, and phonophobia compared with placebo, with NNTps of 3.8, 3.4, and 3.7, respectively. Sumatriptan 6 mg resulted in a small reduction in the number of participants with vomiting compared with placebo, with an NNTp of 40.
Relief of functional disability
Few of the included studies reported relief of functional disability and those that did were inconsistent in both the definition of relief used and the time point at which relief was measured. Three studies (S2BM03; Winner 2006 Study 1 and Study 2) reported complete relief of functional disability (defined as improvement from any disability at baseline to none on a four‐point scale) at two hours after initial dosing, while another (Cady 1993) reported complete relief using the same definition, but at one hour after dosing. Finally three studies (Cady 1991; Cady 1993; Diener 2001) reported partial relief (defined as improvement from moderate or severe disability at baseline to mild or none on a four‐point scale) at one hour after initial dosing. As with associated symptoms, some studies failed to report baseline incidence of functional disability from which relief could be calculated, but did report presence of symptoms one or two hours after treatment.
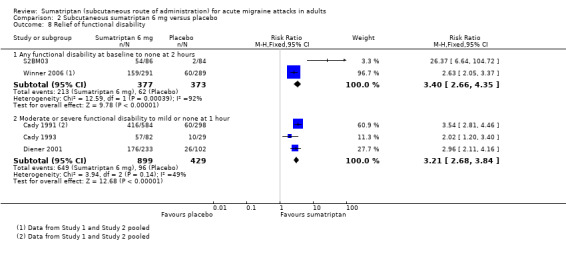
Three studies (750 participants) provided data comparing sumatriptan 6 mg with placebo for the relief of any functional disability at two hours after initial dosing (S2BM03; Winner 2006 Study 1 and Study 2).
The proportion of participants with relief of functional disability at two hours with sumatriptan 6 mg was 56% (213/377; range 55% to 63%).
The proportion of participants with relief of functional disability at two hours with placebo was 17% (62/373; range 2% to 21%).
The relative benefit of treatment compared with placebo was 3.4 (2.7 to 4.4; Analysis 2.8); the NNT was 2.5 (2.2 to 3.0).
2.8. Analysis.
Comparison 2 Subcutaneous sumatriptan 6 mg versus placebo, Outcome 8 Relief of functional disability.
Data were also provided by four studies (1328 participants) comparing sumatriptan 6 mg with placebo for the partial relief of functional disability at one hour after dosing (Cady 1991 Study 1 and Study 2; Cady 1993; Diener 2001).
The proportion of participants with relief of functional disability at two hours with sumatriptan 6 mg was 72% (649/899; range 70% to 76%).
The proportion of participants with relief of functional disability at two hours with placebo was 22% (96/429; 20% to 34%).
The relative benefit of treatment compared with placebo was 3.2 (2.7 to 3.8; Analysis 2.8); the NNT was 2.0 (1.8 to 2.2).
Sumatriptan versus active comparators
One study (Touchon 1996) provided data comparing sumatriptan 6 mg with DHE nasal spray 1 mg for the relief of moderate or severe functional disability at two hours after dosing. Eighty‐two percent of sumatriptan‐treated participants had improved to mild or no functional disability by two hours, compared with 61% of DHE‐treated participants.
One study (Diener 2001) provided data comparing sumatriptan 6 mg with subcutaneous alniditan 1.4 mg and 1.8 mg for the relief of moderate or severe functional disability at one hour after dosing. Seventy‐six percent of sumatriptan‐treated participants had improved to mild or no functional disability by one hour, compared with 71% and 75% of alniditan 1.4 mg‐ and 1.6 mg‐treated participants, respectively.
Presence of functional disability after two hours
We also analysed studies according to the presence of functional disability of either moderate or severe intensity, or of any intensity (on a four‐point scale), one or two hours after treatment, irrespective of whether it was present at baseline, and calculated NNTps. Fewer participants had any functional disability two hours after treatment with sumatriptan 6 mg than with placebo, with a NNTp of 2.9 (Appendix 7).
Adverse events
Details of results for adverse events and withdrawals in individual studies are provided in Appendix 8.
All except four studies (Dahlof 1992; Ferrari 1991; Mushet 1996 Study 1 and Study 2) reported the total number of participants experiencing any adverse event after treatment, although there was significant variability in many details of adverse event reporting in those studies providing data. Most studies appeared to collect data using spontaneous reports in diary cards and at follow‐up review after the end of treatment. The duration over which data were collected was not always specific, and where it was, there were differences between studies. Most studies probably collected data during the 24 hours postdose, but Cady 1991, Diener 1999, and Diener 2001 specified 48 hours; Cady 1993 72 hours; Dahlof 1998 five days; and Cady 1998 collected data over 14 days following treatment. Two studies (SUM40286; SUM40287) specified that adverse events were collected up to the final visit, but did not report when this visit occurred (likely to be more than 24 hours after initial dosing). Finally, two studies (S2BM03; S2BS78) reported that adverse events were collected over several weeks after dosing (up to 14 weeks in one case). The majority of studies reported adverse events regardless of their causal relationship to the study drug, but five studies (Bousser 1993; Henry 1993; Schulman 2000; Winner 2006 Study 1 and Study 2) reported only events considered to be related to the study medication. One study (Visser 1992) reported adverse events for three doses of sumatriptan (1 mg, 2 mg, and 3 mg) combined and therefore could not contribute data to any pooled analyses.
In some studies a second dose of study medication was taken by a proportion of the participants, and in all studies rescue medication was allowed if there was an inadequate response after a given period of time. In four studies (Bates 1994; Russell 1994; S2BM03; S2BS78) adverse event data were collected specifically for participants taking only a single dose of study medication, although for two of these studies (S2BM03; S2BS78) the time period of collection was unclear (and probably mixed, depending on when a second dose was taken). Where the time period of collection was valid, these single‐dose data were used in preference to those for participants taking up to two doses, but it is likely that in all other cases adverse event data continued to be collected after such additional medication.
Despite these inconsistencies, we have included as much data as possible in the adverse event analyses in order to be more inclusive and conservative, but analyses of pooled data on adverse events should be interpreted cautiously.
Treatments were generally described as well tolerated, with most adverse events being of mild or moderate severity and self limiting.
Participants experiencing any adverse event during the 24 hours postdose
Sumatriptan 4 mg versus placebo
Three studies (720 participants) provided data (Mathew 1992; Thomson 1993; Wendt 2006).
The proportion of participants experiencing adverse events within 24 hours with sumatriptan 4 mg was 71% (313/442; range 69% to 83%).
The proportion of participants experiencing adverse events within 24 hours with placebo was 41% (113/278; range 17% to 55%).
The relative harm of treatment compared with placebo was 1.8 (1.6 to 2.2; Analysis 1.5); the NNH was 3.3 (2.7 to 4.4).
1.5. Analysis.
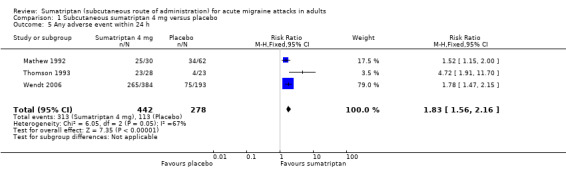
Comparison 1 Subcutaneous sumatriptan 4 mg versus placebo, Outcome 5 Any adverse event within 24 h.
Sumatriptan 6 mg versus placebo
Nine studies (1342 participants) provided data (Akpunonu 1995; Bates 1994; Facchinetti 1995; Gross 1994; Jensen 1995; Mathew 1992; Pfaffenrath 1991; Russell 1994; Sang 2004).
The proportion of participants experiencing adverse events within 24 hours with sumatriptan 6 mg was 44% (341/767; range 33% to 87%).
The proportion of participants experiencing adverse events within 24 hours with placebo was 24% (137/575; range 2% to 55%).
The relative harm of treatment compared with placebo was 2.1 (1.8 to 2.5; Analysis 2.9; Figure 5); the NNH was 4.9 (3.9 to 6.4).
2.9. Analysis.
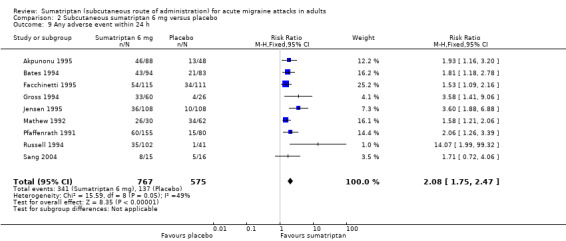
Comparison 2 Subcutaneous sumatriptan 6 mg versus placebo, Outcome 9 Any adverse event within 24 h.
5.
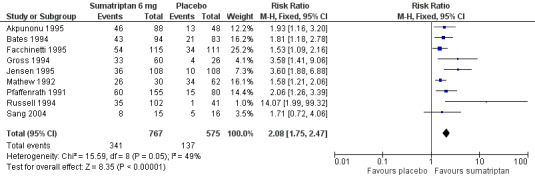
Forest plot of comparison: 2 Subcutaneous sumatriptan 6 mg versus placebo, outcome: 2.9 Any adverse event within 24 h.
Other doses of sumatriptan versus placebo
One study (Mathew 1992) provided data comparing sumatriptan 1 mg, 2 mg, 3 mg, and 8 mg with placebo, but there were insufficient data to carry out pooled analysis of these doses. The proportion of participants experiencing an adverse event within 24 hours of treatment with sumatriptan 1, 2, 3, and 8 mg was 63%, 67%, 80%, and 97%, respectively, while only 55% of placebo‐treated participants experienced an adverse event.
Despite the fact that many studies allowed participants a second dose of study medication, either for recurrence of if they had an inadequate response to the initial dose, only one study provided any data specifically for the incidence of adverse events after two doses of medication. Russell 1994 reported that 34% of participants treated with one dose of sumatriptan 6 mg experienced an adverse event within 24 hours, compared with 25% of participants treated with two doses of sumatriptan 6 mg. In the same study, 2% of participants treated with a single dose of placebo experienced an adverse event, compared with 8% of participants treated with two doses of placebo.
Sumatriptan versus active comparators
Three studies (S2BL99; Sang 2004; Touchon 1996) provided data comparing sumatriptan with an active comparator for the incidence of adverse events within 24 hours of treatment. The three studies used different active comparators so no pooled analysis could be carried out.
S2BL99 provided data comparing sumatriptan 6 mg with oral effervescent ASA 1000 mg + MCP 10 mg. The proportion of participants experiencing an adverse event within 24 hours of treating with sumatriptan was 47%, compared to 21% of participants receiving oral ASA + MCP.
Sang 2004 provided data comparing sumatriptan 6 mg with intravenous LY293558 1.2 mg/kg. The proportion of participants experiencing an adverse event within 24 hours of treating with sumatriptan was 53%, compared to 15% of participants treating with LY293558.
Touchon 1996 provided data comparing sumatriptan 6 mg with DHE nasal spray 1 mg. The proportion of participants experiencing an adverse event within 24 hours of treating with sumatriptan was 43%, compared to 22% of participants treating with DHE nasal spray.
Participants experiencing specific adverse events
Two studies did not report on the incidence of individual adverse events (Bates 1994; Dahlof 1992). The remaining 28 studies reported the incidence of at least one specific adverse event, although there was significant variability in the manner of reporting that further limited the number of studies providing data for pooled analyses. Two studies (Diener 1999; Jensen 1995) reported the number of events, rather than the number of participants experiencing an event, in each treatment arm and therefore did not provide data for analysis. Four studies (Akpunonu 1995; Schulman 2000; Thomson 1993; Touchon 1996) reported the incidence of specific adverse events in the sumatriptan treatment arm but failed to report the incidence in the comparator treatment arm. As discussed previously, the duration over which adverse event data were collected varied between studies and, as with the total incidence of adverse events, 10 studies (Cady 1991 Study 1 and Study 2; Cady 1993; Cady 1998; Dahlof 1998; Diener 1999; Diener 2001; S2BM03; S2BS78; SUM40286; SUM40287) were not included in pooled analyses due to inappropriate collection periods. Finally, one study (Visser 1992) reported specific adverse events for three doses of sumatriptan (1 mg, 2 mg, and 3 mg) combined and therefore could not contribute data to any pooled analyses.
Individual adverse events were reported inconsistently between studies. The majority of studies reported only the most commonly occurring adverse events, for example those occurring in more than 3% of participants in any of the treatment arms, while others used different terms to describe the same or similar events. In order to be as inclusive as possible we have pooled related adverse events into groups (described in detail in Appendix 9). Where one study provided data on more than one event in a particular group, for example reporting both malaise/fatigue and asthenia, we have used the higher incidence in order not to double‐count participants. This will lead to an underestimation of incidence if all those with the less frequent event did not also have the more frequent one.
Again, where studies have provided participants with the option of a second dose of study medication within the adverse event collection period we have used data collected in participants taking a single dose only in preference to data for those taking one or two doses. The small numbers of participants involved in many of the included studies, coupled with the loss of data from participants taking a second dose of study medication (in those studies providing single dose only data) meant that the number of individual adverse events reported in nearly all cases was very low. In addition the loss of participants taking a second dose of study medication was not equal in active treatment and placebo groups, resulting in highly unbalanced treatment and placebo groups in these cases. It was therefore decided that pooled statistical analysis of individual adverse events was invalid, and thus we have simply reported the proportions of participants experiencing specific adverse events within 24 hours of study treatment (Summary of results C).
| Summary of results C: Number of participants experiencing specific adverse events within 24 hours of study treatment in placebo‐controlled studies | ||||
| Studies |
Participants treated |
Treatment (%) | Placebo (%) | |
| Malaise/fatigue/asthenia | ||||
| Sumatriptan 4 mg | 2 | 669 | 3 | 2 |
| Sumatriptan 6 mg | 5 | 593 | 4 | 4 |
| Dizziness/vertigo | ||||
| Sumatriptan 4 mg | 2 | 669 | 10 | 6 |
| Sumatriptan 6 mg | 8 | 993 | 6 | 4 |
| Nausea/vomiting | ||||
| Sumatriptan 4 mg | 2 | 669 | 8 | 10 |
| Sumatriptan 6 mg | 11 | 1667 | 7 | 5 |
| Mouth disorder/disturbance of taste | ||||
| Sumatriptan 4 mg | 2 | 669 | 4 | 1 |
| Sumatriptan 6 mg | 3 | 250 | 6 | 2 |
| Chest pain/symptoms | ||||
| Sumatriptan 4 mg | 2 | 669 | 5 | 1 |
| Sumatriptan 6 mg | 6 | 466 | 4 | 1 |
| Heat sensations/flushing | ||||
| Sumatriptan 4 mg | 2 | 669 | 8 | 4 |
| Sumatriptan 6 mg | 10 | 1149 | 9 | 2 |
| Feeling of heaviness/tightness | ||||
| Sumatriptan 4 mg | 2 | 669 | 6 | 1 |
| Sumatriptan 6 mg | 7 | 962 | 6 | 3 |
| Sweating | ||||
| Sumatriptan 4 mg | 2 | 669 | 1 | 0 |
| Sumatriptan 6 mg | 2 | 318 | 6 | 0 |
| Paraesthesia/numbness | ||||
| Sumatriptan 4 mg | 2 | 669 | 12 | 4 |
| Sumatriptan 6 mg | 10 | 1241 | 7 | 3 |
| Headache | ||||
| Sumatriptan 6 mg | 7 | 727 | 2 | 0 |
| Drowsiness/somnolence | ||||
| Sumatriptan 4 mg | 2 | 669 | 3 | 2 |
| Sumatriptan 6 mg | 4 | 415 | 3 | 3 |
| Neck/back pain | ||||
| Sumatriptan 4 mg | 2 | 669 | 2 | 1 |
| Sumatriptan 6 mg | 5 | 603 | 5 | 1 |
| Throat symptoms | ||||
| Sumatriptan 4 mg | 2 | 669 | 1 | 0 |
| Sumatriptan 6 mg | 3 | 394 | 7 | 0 |
| Injection‐site reaction | ||||
| Sumatriptan 4 mg | 2 | 669 | 45 | 19 |
| Sumatriptan 6 mg | 12 | 1848 | 11 | 6 |
Three studies (S2BL99; Sang 2004; Winner 1996) provided data comparing sumatriptan with an active comparator for the incidence of specific adverse events within 24 hours of treatment. The three studies used different active comparators so no pooled analysis could be carried out.
S2BL99 reported an incidence of 0% to 10% for a range of commonly occurring specific adverse events after treatment with sumatriptan 6 mg, compared with 0% to 7% for the same events after treatment with oral ASA 1000 mg + MCP 10 mg.
Sang 2004 reported an incidence of 2% to 5% for a range of commonly occurring specific adverse events after treatment with sumatriptan 6 mg, compared with 0% to 2% for the same events after treatment with LY293558.
Winner 1996 reported an incidence of 6%, 4%, and 6% for nausea, vomiting, and chest pain, respectively, after treatment with sumatriptan 6 mg, compared with 16%, 7%, and 1% after treatment with subcutaneous DHE 1 mg
Participants experiencing serious adverse events
Sixteen studies did not specifically comment on serious adverse events (Akpunonu 1995; Bates 1994; Cady 1991 Study 1 and Study 2; Cady 1998; Dahlof 1992; Dahlof 1998; Diener 1999; Facchinetti 1995; Ferrari 1991; Gross 1994; Henry 1993; Mathew 1992; Pfaffenrath 1991; Sang 2004; Touchon 1996), 12 studies reported that there were none during the study (Mushet 1996 Study 1 and Study 2; S2BM03; S2BS78; Schulman 2000; SUM40286; SUM40287; Thomson 1993; Visser 1992; Winner 1996; Winner 2006), one study (Jensen 1995) reported no drug‐related serious adverse events, and the remaining six studies (Bousser 1993; Cady 1993; Diener 2001; Russell 1994; S2BL99; Wendt 2006) reported at least one serious adverse event, although most were judged to be unrelated to any study medication.
Sumatriptan versus placebo
Sixteen studies (4741 participants) provided data on sumatriptan of any dose versus placebo (Bousser 1993; Cady 1993; Diener 2001; Mushet 1996 Study 1 and Study 2; Russell 1994; S2BM03; S2BS78; Schulman 2000; SUM40286; SUM40287; Thomson 1993; Visser 1992; Wendt 2006; Winner 2006 Study 1 and Study 2).
The overall incidence of serious adverse events was 0.25% (7/2814) for all doses of sumatriptan (including second doses and rescue medication), and 0.57% (11/1927) for placebo. There were too few events to calculate relative risk or NNH. Further details of individual studies are in Appendix 8.
Sumatriptan versus active comparators
Three studies (1329 participants) provided data on sumatriptan of any dose versus active comparators (Diener 2001; S2BL99; Winner 1996). In all cases there were too few events to calculate relative risk or NNH.
One study (767 participants) comparing sumatriptan with subcutaneous alniditan 1.4 mg and 1.8 mg for the incidence of serious adverse events provided data (Diener 2001). The incidence of serious adverse events was 0% (0/317) for sumatriptan, and 0.22% (1/450) for alniditan.
One study (255 participants) comparing sumatriptan with oral ASA 1000 mg + MCP 10 mg for the incidence of serious adverse events provided data (S2BL99). Neither treatment group reported any serious adverse events.
One study (310 participants) comparing sumatriptan with subcutaneous DHE 1 mg for the incidence of serious adverse events provided data (Winner 1996). Neither treatment group reported any serious adverse events.
Withdrawals due to adverse events
Ten studies did not specifically report on adverse event withdrawals or did not report data for each treatment arm separately. The remaining 25 studies reported the number of withdrawals due to adverse events per treatment group (Bates 1994; Bousser 1993; Cady 1991 Study 1 and Study 2; Cady 1993; Cady 1998; Dahlof 1998; Facchinetti 1995; Henry 1993; Jensen 1995; Mushet 1996 Study 1 and Study 2; Pfaffenrath 1991; Russell 1994; S2BL99; S2BM03; S2BS78; Schulman 2000; SUM40286; SUM40287; Touchon 1996; Visser 1992; Winner 1996; Winner 2006 Study 1 and Study 2).
In studies reporting the occurrence of adverse event withdrawals, 11 reported none (Cady 1998; Dahlof 1998; Henry 1993; Mushet 1996 Study 1 and Study 2; S2BM03; SUM40286; SUM40287; Visser 1992; Winner 2006 Study 1 and Study 2), nine reported an incidence in any treatment arm of less than 2% (Bates 1994; Cady 1991 Study 1 and Study 2; Cady 1993; Pfaffenrath 1991; Russell 1994; Schulman 2000; Touchon 1996; Winner 1996), four reported an incidence of 5% or less (Bousser 1993; Facchinetti 1995; Jensen 1995; S2BL99), and one (S2BS78) reported an incidence of just over 6%.
Sumatriptan versus placebo
Twenty‐two studies (5885 participants) provided data on sumatriptan of any dose versus placebo (Bates 1994; Bousser 1993; Cady 1991 Study 1 and Study 2; Cady 1993; Cady 1998; Dahlof 1998; Facchinetti 1995; Henry 1993; Jensen 1995; Mushet 1996 Study 1 and Study 2; Pfaffenrath 1991; Russell 1994; S2BM03; S2BS78; Schulman 2000; SUM40286; SUM40287; Visser 1992; Winner 2006 Study 1 and Study 2).
The overall incidence of adverse event withdrawal was 1.2% (41/3451) for all doses of sumatriptan (including second doses and rescue medication), and 0.40% (10/2474) for placebo. There were too few events to calculate relative risk or NNH. Further details of individual studies are in Appendix 8.
Sumatriptan versus active comparators
Four studies (1392 participants) provided data on sumatriptan of any dose versus active comparators (Dahlof 1998; S2BL99; Touchon 1996; Winner 1996). In all cases there were too few events to calculate relative risk or NNH.
One study (272 participants) comparing sumatriptan 6 mg with subcutaneous naratriptan 0.5, 1, 2.5, 5, and 10 mg for adverse event withdrawal provided data (Dahlof 1998). No adverse event withdrawals were reported from any of the treatment arms.
One study (255 participants) comparing sumatriptan 6 mg with oral ASA 1000 mg + MCP 10 mg for adverse event withdrawal provided data (S2BL99). The incidence was 4.8% (6/125) for sumatriptan, and 0.77% (1/130) for oral ASA + MCP.
One study (555 participants) comparing sumatriptan 6 mg with DHE nasal spray 1 mg for adverse event withdrawal provided data (Touchon 1996). The incidence was 1.1% (3/278) for sumatriptan, and 0.36% (1/277) for DHE nasal spray.
One study (310 participants) comparing sumatriptan 6 mg with subcutaneous DHE 1 mg for adverse event withdrawal provided data (Winner 1996). The incidence was 0% (0/158) for sumatriptan, and 1.3% (2/152) for subcutaneous DHE.
Discussion
Summary of main results
This review included 35 randomised, double‐blind, controlled studies with 9365 participants. Twenty‐eight studies had only a placebo control, three had only active comparators, and four had both placebo and active comparators. Active comparators were subcutaneous naratriptan, intravenous acetylsalicylic acid lysinate, subcutaneous alniditan, intravenous LY293558, oral effervescent acetylsalicylic acid (ASA) + metoclopramide (MCP), dihydroergotamine (DHE) nasal spray, and subcutaneous DHE. Sumatriptan was studied in doses of 1, 2, 3, 4, 6, and 8 mg in a subcutaneous formulation. Most of the data were for the 6 mg dose. In every study the majority of participants treated established attacks of moderate to severe intensity so no separate analyses were carried out for mild baseline pain.
For all efficacy outcomes, sumatriptan of any dose was superior to placebo and gave clinically useful numbers needed to treat (NNTs). The remarkably consistent response between studies for the primary outcomes, as illustrated by L'Abbé plots (Appendix 10), was not unexpected given the inclusion criteria for the studies and the well‐defined outcomes. The plots for headache relief at one and two hours do, however, show two studies with exceptionally low placebo response rates lying separately to the main body of studies. These two were cross‐over design studies reporting results only for participants completing both phases of the cross‐over. It is not clear what effect the cross‐over design may have on placebo response rates in the second phase following active treatment in the first phase, but it may be that exposure during the first attack to active drug results in reduced response to placebo treatment in the second attack. There was a trend for lower (better) NNTs at higher doses, but significant differences between doses were found only for 4 mg and 6 mg sumatriptan for pain‐free at one hour and for 6 mg and 8 mg sumatriptan for headache relief at one hour. This lack of significant differences is likely to be due to the limited data available for doses of sumatriptan other than 6 mg.
For the IHS‐preferred outcome of pain‐free at two hours, sumatriptan 4 mg and 6 mg compared with placebo gave NNTs of 2.5 and 2.3, respectively, with between 50% and 60% of participants responding after sumatriptan compared to 10% to 15% with placebo. For pain‐free at one hour the NNTs were 3.8, 2.9, and 2.5 for sumatriptan 4 mg, 6 mg, and 8 mg, respectively (about 30% to 45% responders with sumatriptan, 6% with placebo). For headache relief at one hour, sumatriptan 4 mg, 6 mg, and 8 mg compared with placebo gave NNTs of 2.5, 2.2, and 1.7, respectively (about 65% to 80% responders with sumatriptan, 25% with placebo), and for headache relief at two hours sumatriptan 4 mg and 6 mg gave NNTs of 2.1 and 2.1, respectively, when compared with placebo (about 70% to 80% responders with sumatriptan, 20% to 30% with placebo). For sustained pain‐free at 24 hours the NNT for sumatriptan 6 mg was 6.1 (31% responders with sumatriptan, 15% with placebo). The addition of a second dose of sumatriptan 6 mg in the event of an inadequate response at one hour to the initial dose did not significantly improve the NNTs for either pain‐free at two hours or headache relief at two hours.
We carried out sensitivity analyses to assess the impact of small treatment groups and missing data on the primary outcomes. The results from studies in which at least one treatment arm contained fewer than 50 participants were found to differ significantly from studies in which all treatment arms contained more than 50 participants for the pain‐free outcomes. The fact that for one outcome the smaller studies produced a significantly better NNT, and for the other they produced a significantly worse NNT emphasises the considerable effect of random variation on any results generated from very small studies. Similarly, results from studies with substantial missing data were found to be significantly better than those from studies with no missing data for headache relief outcomes. Despite these differences, removing the small studies and those with missing data did not significantly change the overall calculated NNTs due to the fact they contributed only a small proportion of the total data.
Data were available for the use of rescue medication, and for the relief of headache‐associated symptoms and functional disability after treatment with sumatriptan 6 mg. Sumatriptan 6 mg compared with placebo for use of rescue medication within 24 hours of dosing gave a NNTp of 4.8 (27% of sumatriptan‐treated participants requiring rescue medication compared with 48% of placebo‐treated participants). Comparing use of rescue medication at two hours after dosing gave a NNTp of 2.1 (23% of sumatriptan‐treated participants requiring rescue medication compared with 70% of placebo‐treated participants, although it was not clear why this was greater than the proportion of placebo‐treated participants requiring rescue medication within 24 hours. Reported headache‐associated symptoms included nausea, vomiting, photophobia, and phonophobia; vomiting occurred too infrequently for reliable analysis. Sumatriptan 6 mg compared with placebo gave a NNT of 2.4 for relief of nausea at two hours, 2.9 for relief of photophobia, and 3.0 and for phonophobia. Approximately 70% to 75% of participants treated with sumatriptan achieved relief of these symptoms, compared with 35% to 40% of those treated with placebo. Several studies reported relief of associated symptoms at one hour rather than two hours, but no significant differences were found in the NNTs for the two time points. Functional disability was partially relieved (i.e. reduced from moderate or severe at baseline to mild or none at one hour) in 72% of participants treated with sumatriptan 6 mg, and 22% of participants treated with placebo, giving a NNT of 2.0. Functional disability was completely relieved (i.e. reduced from any at baseline to none at two hours) in 56% of participants treated with sumatriptan 6 mg, and 17% of participants treated with placebo, giving a NNT of 2.5.
Analysis of adverse events was compromised by the fact that some studies collected adverse event data over time periods different from the 24‐hour period we specified in our review protocol. Furthermore, studies allowed use of rescue medication for inadequate response (usually after two hours), and many allowed a second dose of study medication for headache recurrence or lack of efficacy, without specifying whether adverse event data continued to be collected from participants who had taken additional medication. In most cases it is likely that it was. With these caveats, we chose to pool as much data as possible. More participants experienced adverse events with sumatriptan than with placebo and data were limited for doses of sumatriptan other than 6 mg. Sumatriptan 4 mg and 6 mg versus placebo gave numbers needed to harm (NNHs) of 3.3 and 4.9, respectively, but there was no significant difference between the two doses. For the most part adverse events were described as mild to moderate in intensity, and self limiting. Serious adverse events were uncommon and only two were possibly related to the study medication: one after treating with sumatriptan 6 mg (participant with known intolerance to ergotamine developed same pattern of symptoms following first dose of sumatriptan) and one after treating with subcutaneous alniditan 1.8 mg (chest pain and prior history of coronary heart disease). Withdrawals due to adverse events were uncommon. In placebo‐controlled studies the rate of adverse event withdrawal after treating with sumatriptan (1.2%) was marginally higher than that after placebo (0.40%). Pooled analyses of individual adverse events were not possible because of the small numbers of participants involved in many of the included studies and the loss of data from participants taking a second dose of study medication. However, the incidence of individual adverse events tended to be higher after treatment with sumatriptan 6 mg than placebo.
There were insufficient data to carry out pooled analyses of sumatriptan versus any active comparator for any of the outcomes of interest for this review. Seven active comparators were used in the included studies: subcutaneous naratriptan, intravenous acetylsalicylic acid lysinate, alniditan, intravenous LY293558, oral effervescent ASA + MCP, DHE nasal spray, and subcutaneous DHE. In general, sumatriptan 6 mg resulted in a higher proportion of treated participants achieving efficacy responses than the active comparators, although the limited data mean that no firm conclusions can be drawn about the relative efficacies.
Overall completeness and applicability of evidence
Included participants suffered from migraine in accordance with IHS criteria (even if not specifically referenced in a few cases), with the majority suffering around one to six attacks per month, and a history of attacks for at least six months, and usually one year. In the majority of studies treated attacks had to be established, with moderate or severe pain intensity, before medication could be taken. The use of prophylactic medication during the study period was variable, with some studies requiring participants to discontinue any prophylactic medication at least two weeks before receiving study medication, while others allowed stable prophylactic medications, and others failed to comment at all. Fourteen studies excluded participants if they had previously taken sumatriptan; some limited this exclusively to subcutaneous sumatriptan and others excluded participants who had any experience with sumatriptan. Two studies required participants to have successfully treated an attack with a 5HT1 agonist in the past, but never to have used a subcutaneous formulation, and one study actually required participants to have regularly used sumatriptan for at least six months before study entry and to experience recurrence of headache in 50% or more of their treated attacks.
Overall there did not appear to be a particular bias towards a certain type of migraine patient, but many studies recruited participants through headache clinics, which may have selected for those with more severe or hard‐to‐treat pain. It is noteworthy that although subcutaneous sumatriptan is most likely to be used by individuals who experience severe nausea and vomiting, and so are unable to take oral medication, this subset of migraineurs were not well represented in the trials. Individuals were carefully screened before study entry and those with certain conditions, particularly cardio‐ or cerebrovascular disease, were excluded from the studies. Other exclusions included pregnant or lactating women, individuals with hepatic disease or who regularly experience vomiting, and individuals who suffer from frequent non‐migraine headaches or basilar, ophthalmic, or hemiplegic migraine. This may mean that the study population is not a reflection of the population most likely to use this formulation of sumatriptan.
While most studies reported IHS‐preferred outcomes, they did not all report all the outcomes of interest for this review so that numbers of participants in any comparison were usually smaller than numbers treated. In addition, there was insufficient evidence to address the sustained efficacy of sumatriptan, an outcome currently thought to be particularly important for acute migraine treatment. Only five studies provided any data on the 24‐hour sustained efficacy of sumatriptan.
Single‐dose studies provide only limited information about adverse events and individual studies are generally underpowered to assess harm, but pooling adverse event data from similar studies may allow more robust estimates for short‐term use. In these studies the number of participants who experienced any adverse events was increased with sumatriptan compared to placebo. However it is important to remember that in many studies rescue medication was permitted if study medication did not provide adequate relief, and this may disproportionately increase rates of adverse events in those taking placebo, due to their increased need over those taking active medication. Furthermore, some studies offered a second dose of study medication if the initial dose did not provide sufficient relief, or in the event of recurrence, and this may disproportionately increase rates of adverse events in those taking two doses of active drug. There were insufficient data to compare confidently the incidence of adverse events after treatment with sumatriptan 6 mg and other doses of sumatriptan. More data on adverse events after the 4 and 8 mg doses of sumatriptan are required to establish whether there is a dose response relationship, and therefore any potential advantage, from a safety point‐of‐view, of using lower doses. Some studies in this review reported data for individual adverse events, but in nearly all cases the studies were underpowered to assess their relative incidence. This was particularly true of those allowing a second dose of study medication in which a significant proportion of the participants were not eligible to contribute to the single‐dose adverse event data. In addition, some studies reported individual events only if they occurred at a specified rate, which differed between studies (> 1% to ≥ 5%), and inevitably meant that some events occurring at lower frequencies were not reported in some studies.
Finally, none of the studies included in this review effectively address the efficacy of subcutaneous sumatriptan to treat migraine headache during the mild pain phase. One study (S2BS78) stated in the protocol that participants should treat at the first sign of headache pain, with the aim of investigating the efficacy, safety, and tolerability of subcutaneous sumatriptan when taken early during a migraine attack. However, only around 35% of participants actually treated a mild headache, meaning that the baseline pain intensity was too heterogeneous to draw any conclusions at all. In clinical practice many people treat their headache during the mild phase, and there is also some evidence that treating attacks in the early stages in beneficial (Gendolla 2008; Pascual 2002), particularly for more common routes of administration such as oral sumatriptan (Derry 2012a).
Very recently a needle‐free delivery system for subcutaneous sumatriptan has been approved for use in the US, and in many countries in Europe, including Denmark, UK, and Germany. Sumavel DosePro uses compressed gas to create a stream of medication that passes through the skin into the subcutaneous tissue. Bioequivalence for this novel method of administration with traditional injected subcutaneous sumatriptan has been demonstrated, but we found no studies specifically addressing its efficacy, safety, and tolerability.
Quality of the evidence
The majority of included studies were of good methodological quality, with only 2/35 deemed to be of low quality (scoring 2 of 5 using the Oxford Quality Scale). However, 29 studies did not adequately describe random sequence generation, 27 studies did not provide information about allocation concealment, and 16 studies did not provide details on the method of blinding. In a number of studies withdrawals and dropouts were not reported adequately by treatment group, and for some outcomes reported denominators differed from the intention‐to‐treat (ITT) population ‐ presumably because some participants failed to record data at that point. Wherever an adequate explanation was not given we have used the ITT denominator if it gave a more conservative estimate; in general the numbers of missing participants were not sufficient to significantly alter the results. Only four studies had at least 200 participants in each treatment arm, a further 16 had between 50 and 200 in one or more treatment arms, and 15 had fewer than 50 participants in all treatment arms. Overall methodological quality of the included studies was acceptable, however treatment group sizes were, in general, small and risk biasing the reported results (Moore 1998).
While most studies used patient diaries and reported some information about adverse events, the outcomes were not always our preferred ones, and the time over which data were collected was frequently not explicit. It is likely that data continued to be collected after intake of rescue medication or a second dose of study medication, so that total dose over the period assessed is uncertain.
Potential biases in the review process
We identified a large amount of data in comparisons with placebo, particularly for the 6 mg dose. Approximately 5000 additional participants would have to have been involved in unpublished trials with zero treatment effect for the NNT for headache relief at two hours to increase above 6 (which we considered the limit of clinical utility in this situation) for the 6 mg dose (Moore 2008). This equates to 10 studies with 500 participants in sumatriptan 6 mg and placebo treatment arms. Similarly, over 6000 additional participants would have to have been involved in unpublished trials with zero treatment effects for the NNT for pain‐free at two hours to increase above 8 (considered to be the limit of clinical utility in this situation). It is unlikely that such a large amount of unidentified data exists, so publication bias is not a concern.
The methods of review were such as to minimise bias due to the review process itself, but use of data from both phases of cross‐over studies and from studies reporting combined data from several attacks may introduce unknown biases. For cross‐over studies a 48‐hour period between qualifying attacks should limit potential for carryover effects.
Sensitivity analyses identified two potential sources of bias in the included studies: size of treatment arms and missing data. Comparing studies which either did not contain at least 50 participants in each treatment arm or had substantial missing data, with larger studies and those with no missing data (i.e. studies with low risk of bias) showed a small, but statistically significant, difference in the estimated effects of treatment for pain‐free and headache relief at one and two hours. Re‐analysing these outcomes using only data from studies with no risk of bias from study size or missing data, however, did not significantly reduce the calculated relative risks and NNTs.
We specified that a minimum of 200 participants in at least two studies were required before carrying out any pooled analysis, but ideally we would need at least 200 participants in each treatment arm where there is an event rate of 50% to be reasonably confident in the size of an effect (Moore 2010). The magnitude of effect for outcomes with fewer participants and/or lower event rates should be interpreted with caution.
Agreements and disagreements with other studies or reviews
Oldman 2002 reviewed all pharmacological treatments for acute migraine, including 14 studies involving subcutaneous sumatriptan, all of which are included here. Of the seven studies involving subcutaneous sumatriptan that were excluded by Oldman et al we have included all but one. The majority of these were excluded because they used doses of sumatriptan other than 6 mg or allowed migraine prophylaxis, both of which are allowed under our inclusion criteria. Results are presented as proportion responding, relative risk, and NNT, and are broadly consistent with those found in this review for the 6 mg dose: NNTs for pain‐free at two hours, headache relief at one hour, and headache relief at two hours are very similar, with the newer estimates tending to be slightly higher (worse), but not significantly different. The considerable amount of additional data included in this review has, however, resulted in tighter confidence intervals for all the calculated NNTs. An attempt was made in Oldman 2002 to address the question of sustained efficacy, and results are presented from two studies on 24‐hour sustained headache relief. Neither of these studies adequately define sustained headache relief which appears to have been calculated from reported recurrence of headache within 24 hours. This does not take into consideration the significant numbers of participants taking rescue medication during this period, without necessarily relapsing back to a full moderate or severe headache (and therefore not categorised as having a recurrence). We considered these data to be unreliable and therefore did not analyse them as part of a sustained efficacy response in this review. Adverse events were not analysed by Oldman et al because of poor reporting, on which we have commented in this review.
Similarly, the results presented here were also largely consistent with those presented in a previous review of triptan use in acute migraine (Gawel 2001) which included data from nine studies comparing subcutaneous sumatriptan with placebo, all of which were included in this review. Again additional data included in this review resulted in slightly reduced estimates of efficacy for the 6 mg dose, particularly for pain‐free and headache relief at one hour outcomes, and tighter confidence intervals.
An earlier review of sumatriptan use for migraine treatment (Tfelt‐Hansen 1998) included data from 13 studies, all of which were included in this review. The results of this review for headache relief at one hour are consistent with those presented here, although once again, the additional data included in our review have increased (worsened) the estimated NNT slightly. In addition Tfelt‐Hansen 1998 analysed the incidence of adverse events after subcutaneous sumatriptan, calculating a NNH of 3.0. This is lower than the estimated NNH from our review (4.9). The discrepancy is the result of more stringent conditions for the analysis of adverse event data that we have used in this review, including using only adverse event data collected within 24 hours of initial dosing, and excluding adverse event data when only events considered related to the study medication were reported. The result of this is that, despite including 17 additional studies in this review, our analysis of adverse events is based on fewer participants.
Authors' conclusions
Implications for practice.
Subcutaneous sumatriptan is an effective treatment for the relief of headache pain, other symptoms associated with migraine, and functional disability, with single doses of 4 mg or more providing clinically useful levels of relief from as early as one hour after administration. Higher doses are effective in more individuals, but at the expense of greater numbers of adverse events. Most events were described as mild and of short duration.
These data suggest that a 4 mg dose (where available) may be a sensible starting dose, with increase to 6 mg if the response is inadequate, and the higher dose is tolerated. There is no evidence that taking a second dose of sumatriptan 6 mg in the event of an inadequate response one hour after the initial dose has a significant impact on headache relief by two hours.
Implications for research.
Given the relatively high cost of the subcutaneous formulation of sumatriptan, future studies should include only those individuals for whom this route is likely to confer significant advantage, namely, those who experience severe nausea and vomiting, and those needing fast relief. They should address sustained outcomes, and consistently report (using standard definitions) relief of associated symptoms and functional disability in this population, together with adverse events.
What's new
| Date | Event | Description |
|---|---|---|
| 29 May 2019 | Amended | Contact details updated. |
| 11 October 2017 | Review declared as stable | No new studies likely to change the conclusions are expected. |
History
Review first published: Issue 2, 2012
| Date | Event | Description |
|---|---|---|
| 1 June 2016 | Review declared as stable | See Published notes. |
Notes
This review is one of a series of reviews on sumatriptan for acute migraine attacks in adults which replaces an earlier Cochrane review of oral sumatriptan (McCrory 2003).
At June 2016, this review has been stabilised. A restricted search in May 2016 did not identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. If appropriate, we will update the review if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Acknowledgements
We would like to acknowledge the helpful comments and editorial expertise of Timothy Steiner, Douglas McCory, and Rebecca Gray, and those who contributed to the various stages of peer review.
Appendices
Appendix 1. Definitions
All terms relating to primary efficacy outcomes are defined according to the effect of the treatment on headache pain, measured using a four‐point pain intensity scale (ranging from 0 to 3 or none, mild, moderate, and severe).
Baseline pain intensity ‐ level of pain participant must be experiencing in order to receive study medication, either 1 (mild pain) or 2/3 (moderate or severe pain).
Pain‐free at two hours (PF2) ‐ number of participants with a pain intensity of 0 (none) at two hours after administration of study medication, expressed as a fraction of the treated participants with the appropriate baseline pain.
Headache relief at two hours (HR2) ‐ number of participants with a reduction in pain intensity from 2/3 (moderate/severe) to 0/1 (none/mild) at two hours after administration of study medication, expressed as a fraction of the treated participants with grade 2/3 baseline pain.
24‐hour sustained headache relief (SHR24) ‐ number of participants with a reduction in pain intensity from 2/3 (moderate/severe) to 0/1 (none/mild) at two hours after administration of study medication which is then sustained between 2 and 24 hours without recurrence of headache or use of rescue medication, expressed as a fraction of the treated participants with grade 2/3 baseline pain.
24‐hour sustained pain‐free (SPF24) ‐ number of participants with a pain intensity of 0 (none) at two hours after administration of study medication which is then sustained between 2 and 24 hours without recurrence of headache or use of rescue medication expressed as a fraction of the treated participants with the appropriate baseline pain.
Use of rescue medication ‐ number of participants requiring the use of additional medication to treat either recurrence of headache or an inadequate response to study medication, provided that the additional medication is not, or does not include, the study drug.
Relief of associated symptoms ‐ number of participants with an absence of a headache‐associated symptom (nausea, vomiting, photophobia, or phonophobia) at one or two hours after administration of study medication, expressed as a fraction of the treated participants for whom the symptom was present at baseline.
Presence of associated symptoms ‐ presence of a headache‐associated symptom (nausea, vomiting, photophobia, or phonophobia) at one or two hours after administration of study medication, expressed as a fraction of all treated participants.
Relief of functional disability ‐ reduction in the level of functional disability, as measured using a four‐point scale, from moderate or severe disability (grade 2/3) at baseline to mild or none (grade 1/0) at one or two hours after administration of study medication, expressed as a fraction of the treated participants with moderate or severe functional disability at baseline.
Complete relief of functional disability ‐ reduction in the level of functional disability, as measured using a four‐point scale, from any disability at baseline to none (grade 0) at one or two hours after administration of study medication, expressed as a fraction of the treated participants with any functional disability at baseline.
Presence of functional disability ‐ presence of functional disability (either moderate or severe in intensity, or any disability) at one or two hours after administration of study medication, expressed as a fraction of all treated participants.
Appendix 2. Search strategy for MEDLINE (via OVID)
Serotonin Agonists/ OR Tryptamines/
(sumatriptan OR Imitrex OR Imigran).mp.
1 OR 2
Headache/ OR exp Headache Disorders/ OR exp Migraine Disorders/
(headach* OR migrain* OR cephalgi* OR cephalalgi*).mp.
4 OR 5
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
OR/7‐14
3 AND 6 AND 15
Appendix 3. Search strategy for EMBASE (via OVID)
Serotonin Agonists/ OR Tryptamines/
(sumatriptan OR Imitrex OR Imigran).mp.
1 OR 2
exp Headache and facial pain
exp Migraine
(headach* OR migrain* OR cephalgi* OR cephalalgi*).mp.
4 OR 5 OR 6
clinical trials.sh.
controlled clinical trials.sh.
randomized controlled trial.sh.
double‐blind procedure.sh.
(clin* adj25 trial*).ab.
((doubl* or trebl* or tripl*) adj25 (blind* or mask*)).ab.
placebo*.ab.
random*.ab.
OR/8‐15
3 AND 7 AND 16
Appendix 4. Search strategy for CENTRAL
MeSH descriptor Serotonin Agonists OR MeSH descriptor Tryptamines
(sumatriptan OR Imitrex OR Imigran):ti,ab,kw
1 OR 2
MeSH descriptor Headache/ OR MeSH descriptor Headache Disorders explode all trees
MeSH descriptor Migraine Disorders explode all trees
(headach* OR migrain* OR cephalgi* OR cephalalgi*):ti,ab,kw
4 OR 5 OR 6
3 AND 7
Limit 8 to Clinical Trials (CENTRAL)
Appendix 5. Summary of outcomes: efficacy
| Study ID | Treatment | Headache relief 1 h | Headache relief 2 h | Pain‐free 1 h | Pain‐free 2 h | Sustained headache relief 24 h | Sustained pain‐free 24 h | Use of rescue medication |
| Akpunonu 1995 | (1) Sumatriptan 6 mg, n = 88 (2) Placebo, n = 48 |
No data | No data | No data | No data | No data | No data | No data |
| Bates 1994 | (1) Sumatriptan 6 mg, n = 90 (88 for efficacy, 47 with moderate or severe baseline pain intensity) (2) Placebo, n = 87 (83 for efficacy, 35 with moderate or severe baseline pain intensity) |
(1) 24/47 (2) 10/35 |
No data | No data | No data | No data | No data | No data |
| Bousser 1993 | (1) Sumatriptan 6 mg, n = 49 (41 for 1st attack efficacy) (2) Placebo, n = 47 (40 for 1st attack efficacy) |
(1) 29/41 (2) 8/40 |
No data | (1) 14/41 (2) 4/40 |
No data | No data | No data | No data |
| Cady 1991 | Study 1 (1) Sumatriptan 6 mg, n = 384 (2) Placebo, n = 190 Study 2 (1) Sumatriptan 6 mg, n = 350 (2) Placebo, n = 180 |
Pooled results for Study 1 and Study 2 (1) 515/734 (2) 81/370 |
No data | Pooled results for Study 1 and Study 2 (1) 356/734 (2) 35/370 |
No data | No data | No data | No data |
| Cady 1993 | (1) Sumatriptan 6 mg, n = 166 (128 treating first attack with moderate or severe baseline pain intensity) (2) Placebo, n = 144 (42 treating first attack with moderate or severe baseline pain intensity) |
1st attack: (1) 100/128 (2) 16/42 |
No data | 1st attack: (1) 41/128 (2) 3/42 |
No data | (1) 39/128 (2) 5/42 |
(1) 26/128 (2) 5/42 |
No data |
| Cady 1998 | (1) Sumatriptan 6 mg, n = 67 (2) Placebo, n = 68 (65 for efficacy) |
No data | No data | No data | No data | No data | No data | At 24 h: (1) 5/67 (2) 20/65 |
| Dahlof 1992 | (1) Sumatriptan 8 mg, n = 27 (2) Placebo, n = 27 |
(1) 23/27 (2) 3/27 |
(1) 23/27 (2) 9/27 |
No data | (1) 17/27 (2) 0/27 |
No data | No data | At 2 h: (1) 3/27 (2) 24/27 |
| Dahlof 1998 | (1) Sumatriptan 6 mg, n = 47 (2) Naratriptan 0.5 mg, n = 60 (3) Naratriptan 1 mg, n = 55 (4) Naratriptan 2.5 mg, n = 42 (5) Naratriptan 5 mg, n = 34 (6) Naratriptan 10 mg, n = 34 (7) Placebo, n = 63 |
(1) 41/47 (2) 36/60 (3) 35/55 (4) 34/42 (5) 29/34 (6) 26/34 (7) 26/63 |
(1) 42/47 (2) 39/60 (3) 41/55 (4) 35/42 (5) 32/34 (6) 31/34 (7) 26/63 |
No data | (1) 26/47 (2) 18/60 (3) 24/55 (4) 25/42 (5) 27/34 (6) 30/34 (7) 11/63 |
No data | No data | At 24 h: (1) 2/47 (2) 21/60 (3) 12/55 (4) 5/42 (5) 2/34 (6) 1/34 (7) 22/63 |
| Diener 1999 | (1) Sumatriptan 6 mg, n = 114 (2) Intravenous acetylsalicylic acid lysinate 1.8 g, n = 119 (3) Placebo, n = 42 |
(1) 84/114 (2) 71/119 (3) 8/42 |
(1) 104/114 (2) 88/119 (3) 10/42 |
No data | (1) 87/114 (2) 52/119 (3) 6/42 |
No data | No data | At 24 h: (1) 2/114 (2) 5/119 (3) 7/42 |
| Diener 2001 | (1) Sumatriptan 6 mg, n = 317 (2) Alniditan 1.4 mg, n = 309 (3) Alniditan 1.8 mg, n = 141 (4) Placebo, n = 157 (156 for efficacy) |
(1) 250/317 (2) 231/309 (3) 114/141 (4) 50/156 |
(1) 276/317 (2) 250/309 (3) 120/141 (4) 59/156 |
No data | (1) 209/317 (2) 174/309 (3) 87/141 (4) 22/156 |
No data | No data | At 24 h: (1) 155/317 (2) 142/309 (3) 65/141 (4) 123/156 |
| Facchinetti 1995 | (1) Sumatriptan 6 mg, n = 115 (77 for first dose efficacy with moderate or severe baseline pain intensity) (2) Placebo, n = 111 (92 for first dose efficacy with moderate or severe baseline pain intensity) |
(1) 54/77 (2) 20/92 |
(1) 56/77 (2) 27/92 |
(1) 25/77 (2) 9/92 |
(1) 40/77 (2) 12/92 |
No data | No data | At 2 h: (1) 18/77 (2) 52/92 |
| Ferrari 1991 | (1) Sumatriptan 6 mg, n = 423 (422 for efficacy) (2) Sumatriptan 8 mg, n = 110 (109 for efficacy) (3) Placebo, n = 106 (105 for efficacy) |
(1) 308/422 (2) 86/109 (3) 26/106 |
No data | (1) 186/422 (2) 55/109 (3) 8/106 |
No data | No data | No data | No data |
| Gross 1994 | (1) Sumatriptan 6 mg, n = 60 (48 with moderate or severe baseline pain intensity) (2) Placebo, n = 26 (18 with moderate or severe baseline pain intensity) |
(1) 42/48 (2) 2/18 |
No data | No data | No data | No data | No data | No data |
| Henry 1993 | (1) Sumatriptan 6 mg, n = 37 (2) Placebo, n = 39 |
(1) 22/37 (2) 8/39 |
No data | (1) 12/37 (2) 4/39 |
No data | No data | No data | No data |
| Jensen 1995 | (1) Sumatriptan 6 mg, n = 117 attacks (108 for cross‐over efficacy analysis) (2) Placebo, n = 109 attacks (108 for cross‐over efficacy analysis) |
(1) 66/108 (2) 6/108 |
(1) 73/108 (2) 11/108 |
(1) 33/108 (2) 1/108 |
No data | No data | No data | At 2 h: (1) 24/108 (2) 81/108 |
| Mathew 1992 | (1) Sumatriptan 1 mg, n = 30 (2) Sumatriptan 2 mg, n = 30 (3) Sumatriptan 3 mg, n = 30 (4) Sumatriptan 4 mg, n = 30 (5) Sumatriptan 6 mg, n = 30 (6) Sumatriptan 8 mg, n = 30 (7) Placebo, n = 62 |
(1) 13/30 (2) 17/30 (3) 17/30 (4) 15/30 (5) 22/30 (6) 24/30 (7) 15/62 |
(1) 12/30 (2) 14/30 (3) 17/30 (4) 18/30 (5) 21/30 (6) 26/30 (7) 14/62 |
(1) 4/30 (2) 1/30 (3) 7/30 (4) 5/30 (5) 12/30 (6) 10/30 (7) 2/62 |
(1) 6/30 (2) 3/30 (3) 8/30 (4) 10/30 (5) 18/30 (6) 16/30 (7) 2/62 |
No data | No data | At 4 h: (1) 19/30 (2) 15/30 (3) 14/30 (4) 13/30 (5) 10/30 (6) 10/30 (7) 48/62 |
| Mushet 1996 | Study 1 (1) Sumatriptan 6 mg, n = 40 (2) Placebo, n = 39 Study 2 (1) Sumatriptan 6 mg, n = 40 (2) Placebo, n = 39 |
Study 1 (1) 28/40 (2) 10/40 Study 2 (1) 30/39 (2) 12/39 |
Study 1 (1) 29/40 (2) 11/40 Study 2 (1) 31/39 (2) 14/39 |
Study 1 (1) 12/40 (2) 0/40 Study 2 (1) 16/39 (2) 2/39 |
Study 1 (1) 24/40 (2) 4/40 Study 2: (1) 22/39 (2) 5/39 |
No data | No data | No data |
| Pfaffenrath 1991 | (1) Sumatriptan 6 mg, n = 155 (147 with moderate or severe baseline pain intensity) (2) Placebo, n = 80 (69 with moderate or severe baseline pain intensity) |
(1) 99/147 (2) 17/69 |
No data | (1) 40/147 (2) 3/69 |
No data | No data | No data | No data |
| Russell 1994 | (1) Sumatriptan 6 mg, n = 209 (2) Placebo, n = 209 |
No data | No data | No data | No data | No data | No data | No data |
| S2BL99 | (1) Sumatriptan 6 mg, n = 125 (122 with moderate or severe baseline pain intensity for attack 1) (2) Oral ASA 1000 mg + MCP 10 mg, n = 130 (125 with moderate or severe baseline pain intensity for attack 1) |
1st attack: (1) 87/122 (2) 57/125 |
1st attack: (1) 99/122 (2) 79/125 |
1st attack: (1) 55/122 (2) 26/125 |
1st attack: (1) 75/122 (2) 46/125 |
No data | No data | 1st attack, up to 24 h: (1) 27/125 (2) 45/130 |
| S2BM03 | (1) Sumatriptan 6 mg (+ placebo at 4 h), n = 106 (90 for cross‐over efficacy analysis, of which 87 had moderate or severe baseline pain intensity) (2) Placebo (+ sumatriptan 6 mg at 4 h), n = 106 (90 for cross‐over efficacy analysis, of which 81 had moderate or severe baseline pain intensity) |
(1) 64/87 (2) 7/81 |
(1) 72/87 (2) 8/81 |
(1) 41/87 (2) 1/81 |
(1) 56/87 (2) 3/81 |
No data | No data | No data |
| S2BS78 | (1) Sumatriptan 6 mg, n = 236 (2) Placebo, n = 117 |
No data | No data | No data | No data | No data | No data | No data |
| Sang 2004 | (1) Sumatriptan 6 mg, n = 15 (2) Intravenous LY293558 1.2 mg/kg, n = 13 (3) Placebo, n = 16 (15 with moderate or severe baseline pain intensity) |
(1) 11/15 (2) 9/13 (3) 2/15 |
(1) 13/15 (2) 9/13 (3) 4/15 |
(1) 4/15 (2) 4/13 (3) 1/15 |
(1) 9/15 (2) 7/13 (3) 1/15 |
No data | No data | At 2 h: (1) 2/15 (2) 4/13 (3) 14/16 |
| Schulman 2000 | (1) Sumatriptan 6 mg, n = 79 (76 for efficacy) (2) Placebo, n = 40 |
(1) 48/76 (2) 13/40 |
No data | No data | No data | No data | No data | At 24 h: (1) 4/76 (2) 4/40 |
| SUM40286 | (1) Sumatriptan 6 mg, n = 146 (145 for efficacy) (2) Placebo, n = 153 (152 for efficacy) |
(1) 95/145 (2) 53/152 |
(1) 104/145 (2) 62/152 |
(1) 49/145 (2) 17/152 |
(1) 70/145 (2) 28/152 |
No data | (1) 47/145 (2) 22/152 |
No data |
| SUM40287 | (1) Sumatriptan 6 mg, n = 149 (148 for efficacy) (2) Placebo, n = 139 |
(1) 105/148 (2) 47/139 |
(1) 114/148 (2) 44/139 |
(1) 64/148 (2) 9/139 |
(1) 84/148 (2) 26/139 |
No data | (1) 51/148 (2) 21/139 |
No data |
| Thomson 1993 | (1) Sumatriptan 4 mg, n = 28 (2) Placebo, n = 23 (22 for efficacy) |
No data | No data | No data | No data | No data | No data | No data |
| Touchon 1996 | (1) Sumatriptan 6 mg, n = 278 (145 treated first attack, 266 in cross‐over analysis) (2) DHE nasal spray 1 mg, n = 277 (144 treated first attack, 266 in cross‐over analysis) |
(1) 189/266 (2) 90/266 |
(1) 215/266 (2) 138/266 |
(1) 125/266 (2) 35/266 |
(1) 176/266 (2) 82/266 |
(1) 144/266 (2) 104/266 |
No data | No data |
| Visser 1992 | (1) Sumatriptan 1 mg, n = 170 (2) Sumatriptan 2 mg, n = 171 (3) Sumatriptan 3 mg, n = 172 (4) Placebo, n = 172 |
No data | No data | No data | No data | No data | No data | No data |
| Wendt 2006 | (1) Sumatriptan 4 mg, n = 384 (381 with moderate or severe baseline pain intensity) (2) Placebo, n = 193 (191 with moderate or severe baseline pain intensity) |
(1) 256/381 (2) 49/191 |
(1) 268/381 (2) 42/191 |
(1) 129/381 (2) 14/191 |
(1) 191/381 (2) 21/191 |
No data | No data | At 24 h: (1) 84/384 (2) 86/193 |
| Winner 1996 | (1) Sumatriptan 6 mg, n = 158 (150 for efficacy) (2) Subcutaneous dihydroergotamine (DHE) mesylate 1 mg, n = 152 (145 for efficacy) |
(1) 117/150 (2) 82/145 |
(1) 128/150 (2) 106/145 |
No data | No data | No data | No data | No data |
| Winner 2006 | Study 1 (1) Sumatriptan 6 mg, n = 146 (145 for efficacy, 144 with moderate or severe baseline pain intensity) (2) Placebo, n = 153 (152 for efficacy, 151 with moderate or severe baseline pain intensity) Study 2 (1) Sumatriptan 6 mg, n = 149 (148 for efficacy) (2) Placebo, n = 139 |
Pooled results for Study 1 and Study 2 (1) 192/292 (2) 95/290 |
Study 1 (1) 103/144 (2) 61/151 Study 2 (1) 114/148 (2) 44/139 |
No data | Study 1 (1) 70/144 (2) 28/151 Study 2 (1) 84/148 (2) 26/139 |
No data | Study 1 (1) 47/144 (2) 22/151 Study 2 (1) 51/148 (2) 21/139 |
No data |
Appendix 6. Summary of results: Sensitivity analyses for sumatriptan 6 mg versus placebo
| Studies |
Attacks treated |
Treatment (%) |
Placebo (%) |
Relative risk (95% CI) |
NNT (95% CI) |
P for difference | |
| Effect of size | |||||||
| Pain‐free at 2 hours (in studies containing ≥ 50 participants in each treatment arm) | 7 | 1976 | 58 | 16 | 3.6 (3.0 to 4.2) | 2.4 (2.2 to 2.7) |
z = 3.195 P = 0.001 |
| Pain‐free at 2 hours (in studies containing 1 or more treatment arm with < 50 participants) | 6 | 546 | 65 | 11 | 5.3 (3.7 to 7.6) | 1.9 (1.6 to 2.1) | |
| Pain‐free at 1 hour (in studies containing ≥ 50 participants in each treatment arm) | 9 | 2985 | 43 | 7 | 5.5 (4.5 to 6.9) | 2.9 (2.7 to 3.1) |
z = 2.210 P = 0.027 |
| Pain‐free at 1 hour (in studies containing 1 or more treatment arm with < 50 participants) | 7 | 607 | 34 | 6 | 5.6 (3.4 to 9.3) | 3.6 (3.0 to 4.5) | |
| Headache relief at 1 hour (in studies containing ≥ 50 participants in each treatment arm) | 12 | 4040 | 71 | 26 | 2.7 (2.5 to 3.0) | 2.2 (2.1 to 2.4) |
z = 0.145 P = 0.881 |
| Headache relief at 1 hour (in studies containing 1 or more treatment arm with < 50 participants) | 12 | 1137 | 73 | 27 | 2.7 (2.3 to 3.1) | 2.2 (2.0 to 2.5) | |
| Headache relief at 2 hours (in studies containing ≥ 50 participants in each treatment arm) | 8 | 2192 | 78 | 31 | 2.5 (2.2 to 2.7) | 2.1 (2.0 to 2.3) | z = 1.806 P = 0.070 |
| Headache relief at 2 hours (in studies containing 1 or more treatment arm with < 50 participants) | 6 | 546 | 84 | 30 | 2.7 (2.2 to 3.4) | 1.9 (1.6 to 2.1) | |
| Effect of missing data | |||||||
| Pain‐free at 1 hour (in studies with no missing data) | 14 | 3208 | 41 | 8 | 5.0 (4.1 to 6.1) | 3.0 (2.8 to 3.3) |
z = 0.908 P = 0.363 |
| Pain‐free at 1 hour (in studies with missing data) | 2 | 384 | 38 | 1 | 36 (8.9 to 140) | 2.7 (2.3 to 3.3) | |
| Headache relief at 1 hour (in studies with no missing data) | 22 | 4793 | 71 | 28 | 2.6 (2.4 to 2.8) | 2.3 (2.2 to 2.5) |
z = 4.068 P < 0.00006 |
| Headache relief at 1 hour (in studies with missing data) | 2 | 384 | 67 | 7 | 9.6 (5.7 to 16) | 1.7 (1.5 to 1.9) | |
| Headache relief at 2 hours (in studies with no missing data) | 12 | 2354 | 80 | 34 | 2.3 (2.1 to 2.5) | 2.2 (2.1 to 2.4) |
z = 4.520 P < 0.00006 |
| Headache relief at 2 hours (in studies with missing data) | 2 | 384 | 74 | 10 | 7.4 (4.8 to 11) | 1.6 (1.4 to 1.8) |
Appendix 7. Associated symptoms: presence two hours after treatment
| Associated symptoms: symptom present 2 hours after taking study medication in placebo controlled studies | ||||||
| Intervention | Studies | Attacks treated | Treatment (%) | Placebo (%) | Relative risk (95% CI) | NNTp (95% CI) |
| Nausea | ||||||
| Sumatriptan 6 mg | 9 | 1879 | 17 | 43 | 0.39 (0.33 to 0.46) | 3.8 (3.3 to 4.4) |
| Vomiting | ||||||
| Sumatriptan 6 mg | 8 | 1710 | 5 | 8 | 0.43 (0.29 to 0.63) | 40 (21 to 1000) |
| Photophobia | ||||||
| Sumatriptan 6 mg | 5 | 1324 | 26 | 55 | 0.49 (0.42 to 0.56) | 3.4 (2.9 to 4.1) |
| Phonophobia | ||||||
| Sumatriptan 6 mg | 5 | 1324 | 22 | 49 | 0.46 (0.39 to 0.54) | 3.7 (3.2 to 4.6) |
| Any functional disability | ||||||
| Sumatriptan 6 mg | 6 | 1455 | 39 | 73 | 0.53 (0.48 to 0.59) | 2.9 (2.6 to 3.4) |
Appendix 8. Summary of outcomes: adverse events and withdrawals
| Study ID | Treatment | > 1 dose of study medication available | Any AE | Specific AEs | Serious AEs | AE withdrawal | Other withdrawals/exclusions |
| Akpunonu 1995 | (1) Sumatriptan 6 mg, n = 88 (2) Placebo, n = 48 |
No | Within 24 hours: (1) 46/88 (2) 13/48 |
No data | No data | No data | No data |
| Bates 1994 | (1) Sumatriptan 6 mg, n = 90 (94 for safety) (2) Placebo, n = 87 (83 for safety) |
Yes | Within 24 hours: (1) 43/94 (2) 21/83 |
No data | No data | (1) 1/94 (2) 0/83 |
4 participants originally randomised to placebo took only the second injection (sumatriptan) and were included in safety analysis for sumatriptan 6 participants were excluded from the efficacy analyses either because they took the treatments in the wrong order or because they had inadequate diary booklet information |
| Bousser 1993 | (1) Sumatriptan 6 mg, n = 49 (92 for safety) (2) Placebo, n = 47 (89 for efficacy) |
Yes | Drug‐related, within 24 hours: (1) 34/92 (2) 2/89 |
Drug‐related: Injection‐site reaction: (1) 12/92; (2) 0/89 Paraesthesiae: (1) 9/92; (2) 0/89 Flushing: (1) 7/92; (2) 0/89 Palpitations/sweating: (1) 6/92; (2) 1/89 Digestive disorders: (1) 4/92; (2) 1/89 Vertigo/malaise: (1) 4/92; (2) 0/89 Nervousness, anxiety, drowsiness: (1) 3/92; (2) 2/89 |
(1) 0/92 (2) 2/89 |
(1) 0/92 (2) 2/89 |
Of the 96 participants treating the first attack, 12 did not treat a second attack and were excluded from the cross‐over efficacy population: 9 did not experience a second attack 3 withdrew after first attack (2 for adverse events, 1 for pretreatment biological abnormality) |
| Cady 1991 | Study 1 (1) Sumatriptan 6 mg, n = 384 (2) Placebo, n = 190 Study 2 (1) Sumatriptan 6 mg, n = 350 (2) Placebo, n = 180 |
Yes | Within 48 hours (pooled results for study 1 and study 2): (1) 622/734 (2) 197/370 |
Reported by ≥ 1% of participants after single dose only (pooled results for study 1 and study 2): Flushing: (1) 36/547; (2) 9/370 Hypertension: (1) 4/547; (2) 3/370 Throat symptoms: (1) 18/547; (2) 2/370 Disease of nasal cavity/sinuses: (1) 12/547; (2) 0/370 Nausea and/or vomiting: (1) 68/547; (2) 52/370 Abdominal discomfort: (1) 7/547; (2) 3/370 Injection‐site reaction: (1) 321/547; (2) 88/370 Pressure sensation: (1) 39/547; (2) 6/370 Feeling of heaviness: (1) 40/547; (2) 4/370 Chest symptoms: (1) 30/547; (2) 5/370 Disorder of mouth/tongue: (1) 27/547; (2) 17/370 Weakness: (1) 27/547; (2) 1/370 Neck pain/stiffness: (1) 26/547; (2) 2/370 Feeling of tightness: (1) 28/547; (2) 1/370 Anxiety: (1) 6/547; (2) 2/370 Drowsiness/sedation: (1) 15/547; (2) 8/370 Dizziness/vertigo: (1) 65/547; (2) 16/370 Malaise/fatigue: (1) 6/547; (2) 3/370 Disturbance of taste: (1) 15/547; (2) 11/370 Burning sensation: (1) 41/547; (2) 1/370 Numbness: (1) 25/547; (2) 8/370 Tingling: (1) 74/547; (2) 11/370 Warm/hot sensation: (1) 59/547; (2) 13/370 Sweating: (1) 7/547; (2) 3/370 |
No data | Pooled results for study 1 and study 2: (1) 6/734 (2) 1/370 |
No data |
| Cady 1993 | (1) Sumatriptan 6 mg, n = 166 (2) Placebo, n = 144 |
No | Within 72 hours (data from 4 attacks pooled): (1) 152/166 (2) 62/144 |
Data from 4 attacks pooled: Injection‐site reaction: (1) 131/166; (2) 34/144 Nausea and/or vomiting: (1) 38/166; (2) 14/144 Tingling: (1) 38/166; (2) 2/144 Warm/hot sensation: (1) 31/166; (2) 1/144 Chest symptoms: (1) 26/166; (2) 0/144 Flushing: (1) 25/166; (2) 2/144 Pressure sensation: (1) 23/166; (2) 3/144 Feeling of tightness: (1) 22/166; (2) 0/144 Migraine: (1) 21/166; (2) 4/144 Disorder of mouth/tongue: (1) 20/166; (2) 3/144 Numbness: (1) 9/166; (2) 3/144 Disease of nasal cavity/sinuses: (1) 8/166; (2) 2/144 Hypertension: (1) 6/166; (2) 2/144 |
Data from 4 attacks pooled: (1) 5/166 (2) 3/144 |
Data from 4 attacks pooled: (1) 3/166 (2) 0/144 |
47 participants withdrawn due to failure to treat all 4 attacks in cross‐over study |
| Cady 1998 | (1) Sumatriptan 6 mg, n = 67 (2) Placebo, n = 68 |
Yes | Within 14 days: (1) 35/67 (2) 14/68 |
Experienced by > 5% of participants in sumatriptan group Warm or hot sensation: (1) 10/67; (2) 1/68 Nausea and vomiting: (1) 7/67; (2) 2/68 Dizziness: (1) 5/67; (2) 2/68 Injection‐site reaction: (1) 5/67; (2) 2/68 Pressure sensation: (1) 5/67; (2) 0/68 Chest tightness: (1) 4/67; (2) 0/68 Tingling: (1) 4/67; (2) 0/68 |
No data | (1) 0/67 (2) 0/68 |
3 participants randomised to placebo were excluded from the efficacy analyses: 1 failed to return to the clinic 2 did not use treatment in accordance with the study protocol |
| Dahlof 1992 | (1) Sumatriptan 8 mg, n = 27 (2) Placebo, n = 27 |
No | No data | No data | No data | No data | No data |
| Dahlof 1998 | (1) Sumatriptan 6 mg, n = 47 (2) Naratriptan 0.5 mg, n = 60 (3) Naratriptan 1 mg, n = 55 (4) Naratriptan 2.5 mg, n = 42 (5) Naratriptan 5 mg, n = 34 (6) Naratriptan 10 mg, n = 34 (7) Placebo, n = 63 |
No | Within 5 days: (1) 25/47 (2) 20/60 (3) 16/55 (4) 18/42 (5) 20/34 (6) 24/34 (7) 14/63 |
Occurring in ≥ 7 participants in any naratriptan treatment group: Malaise/fatigue: (1) 4/47; (2) 4/60; (3) 2/55; (4) 4/42; (5) 6/34; (6) 12/34; (7) 2/63 Feeling of heaviness: (1) 8/47; (2) 3/60; (3) 3/55; (4) 4/42; (5) 6/34; (6) 7/34; (7) 3/63 Injection‐site reaction: (1) 6/47; (2) 2/60; (3) 3/55; (4) 3/42; (5) 4/34; (6) 7/34; (7) 3/63 Warm/hot sensation: (1) 4/47; (2) 2/60; (3) 1/55; (4) 2/42; (5) 3/34; (6) 9/34; (7) 1/63 Tingling: (1) 4/47; (2) 2/60; (3) 3/55; (4) 1/42; (5) 2/34; (6) 6/34; (7) 2/63 Dizziness/vertigo: (1) 2/47; (2) 2/60; (3) 3/55; (4) 0/42; (5) 2/34; (6) 3/34; (7) 1/63 Pressure sensation: (1) 3/47; (2) 0/60; (3) 1/55; (4) 1/42; (5) 3/34; (6) 4/34; (7) 0/63 Nausea/vomiting: (1) 1/47; (2) 1/60; (3) 2/55; (4) 2/42; (5) 0/34; (6) 3/34; (7) 5/63 Chest pressure: (1) 2/47; (2) 1/60; (3) 0/55; (4) 3/42; (5) 0/34; (6) 3/34; (7) 1/63 |
No data | (1) 0/47 (2) 0/60 (3) 0/55 (4) 0/42 (5) 0/34 (6) 0/34 (7) 0/63 |
No data |
| Diener 1999 | (1) Sumatriptan 6 mg, n = 114 (116 for safety) (2) Intravenous acetylsalicylic acid lysinate 1.8 g, n = 119 (3) Placebo, n = 42 (43 for safety) |
No | Within 48 hours: (1) 38/116 (2) 9/119 (3) 4/43 |
Only reported as number of events occurring rather than number of participants with specific AEs | No data | No data | 3 participants (2 from sumatriptan group and 1 from placebo group) excluded from efficacy analyses due to violation of exclusion criteria |
| Diener 2001 | (1) Sumatriptan 6 mg, n = 317 (2) Alniditan 1.4 mg, n = 309 (3) Alniditan 1.8 mg, n = 141 (4) Placebo, n = 157 |
No | Within 48 hours: (1) 210/317 (2) 214/309 (3) 91/141 (4) 62/157 |
Occurring in > 5% of all participants: Headache: (1) 74/317; (2) 60/309; (3) 38/141; (4) 5/157 Paraesthesia: (1) 40/317; (2) 59/309; (3) 27/141; (4) 9/157 Fatigue: (1) 46/317; (2) 47/309; (3) 21/141; (4) 10/157 Chest pain: (1) 28/317; (2) 36/309; (3) 25/141; (4) 2/157 Application site reaction: (1) 46/317; (2) 22/309; (3) 6/141; (4) 10/157 Change in temperature sensation: (1) 29/317; (2) 17/309; (3) 11/141; (4) 8/157 Nausea: (1) 29/317; (2) 19/309; (3) 11/141; (4) 2/157 |
(1) 0/317 (2) 0/309 (3) 1/141 (4) 1/157 |
No data | 3 participants withdrew before trial completion: 1 subject on alniditan 1.4 mg and 1 on sumatriptan were lost to follow‐up, whilst 1 on placebo was noncompliant All participants included in both efficacy and safety analyses |
| Facchinetti 1995 | (1) Sumatriptan 6 mg, n = 115 (2) Placebo, n = 111 |
Yes | Within 24 hours: (1) 54/115 (2) 34/111 |
Reported most frequently: Dizziness/vertigo: (1) 12/115; (2) 5/111 Nausea/vomiting: (1) 10/115; (2) 3/111 Paraesthesia: (1) 10/115; (2) 3/111 Tingling: (1) 8/115; (2) 3/111 Warm/hot sensations: (1) 8/115; (2) 1/111 Injection‐site reaction: (1) 7/115; (2) 5/111 Throat symptoms: (1) 7/115; (2) 1/111 Neck pain/stiffness: (1) 5/115; (2) 2/111 Sweating: (1) 5/115; (2) 0/111 Pressure sensation: (1) 2/115; (2) 5/111 |
No data | (1) 3/115 (2) 2/111 |
47 participants were excluded from the efficacy analyses due to treating a migraine attack outside of the menstrual window (‐3 to +5 days): 32 from the sumatriptan group and 15 from the placebo group A further 10 participants were excluded from primary efficacy analyses due to insufficient baseline pain intensity: 6 from the sumatriptan group and 4 from the placebo group |
| Ferrari 1991 | (1) Sumatriptan 6 mg, n = 423 (2) Sumatriptan 8 mg, n = 110 (3) Placebo, n = 106 |
Yes | No data | Most frequently reported after a single dose only: Injection‐site reaction: (1) 26/203; (2) 6/60; (3) 0/13 Nausea or vomiting: (1) 12/203; (2) 4/60; (3) 0/13 Flushing: (1) 10/203; (2) 4/60; (3) 2/13 Warm or hot sensation: (1) 22/203; (2) 6/60; (3) 0/13 Feeling of heaviness: (1) 24/203; (2) 10/60; (3) 2/13 Pressure sensation: (1) 18/203; (2) 4/60; (3) 1/13 Weakness: (1) 6/203; (2) 4/60; (3) 1/13 Drowsiness/sedation: (1) 2/203; (2) 0/60; (3) 0/13 Dizziness or vertigo: (1) 2/203; (2) 3/60; (3) 0/13 Malaise or fatigue: (1) 8/203; (2) 4/60; (3) 2/13 Paraesthesia (1) 6/203; (2) 4/60; (3) 0/13 Tingling: (1) 6/203; (2) 3/60; (3) 1/13 Headache: (1) 1/203; (2) 2/60; (3) 0/13 |
No data | No data | 3 participants were excluded from efficacy analyses due to insufficient baseline pain intensity or taking other medications before or during the study 2 other participants were excluded from efficacy analyses after 1 hour due to erroneous treatment with open‐label sumatriptan at 1 hour |
| Gross 1994 | (1) Sumatriptan 6 mg, n = 60 (2) Placebo, n = 26 |
Yes | Within 24 hours: (1) 33/60 (2) 4/26 |
Most commonly reported after a single dose only: Injection site: (1) 4/40; (2) 0/2 Nausea and vomiting: (1) 2/40; (2) 0/2 Headache: (1) 2/40; (2) 0/2 Flushing: (1) 3/40; (2) 0/2 Burning sensation: (1) 1/40; (2) 0/2 Tingling: (1) 3/40; (2) 0/2 Chest symptoms: (1) 2/40; (2) 0/2 Numbness: (1) 3/40; (2) 0/2 Paraesthesia: (1) 3/40; (2) 0/2 Warm/hot sensation: (1) 3/40; (2) 0/2 |
No data | 1 participant discontinued (group not reported) | 1 participant discontinued treatment because of a dislike of injections |
| Henry 1993 | (1) Sumatriptan 6 mg, n = 37 (2) Placebo, n = 39 |
Yes | Drug‐related, within 24 hours: (1) 10/37 (2) 1/39 |
Notified as drug‐related: Flushing: (1) 1/37; (2) 0/39 Injection‐site reaction: (1) 1/37; (2) 1/39 Sickness/vertigo/ hypothymia: (1) 6/37; (2) 0/39 Paraesthesia: (1) 0/37; (2) 1/39 Drowsiness: (1) 1/37; (2) 0/39 Thoracic discomfort/laryngeal oppression: (1) 4/37; (2) 0/39 Muscular weakness: (1) 1/37; (2) 0/39 Nausea: (1) 2/37; (2) 0/39 Headache: (1) 1/37; (2) 0/39 |
No data | (1) 0/37 (2) 0/39 |
No data |
| Jensen 1995 | (1) Sumatriptan 6 mg, n = 117 attacks (108 for cross‐over efficacy analysis) (2) Placebo, n = 109 attacks (108 for cross‐over efficacy analysis) |
Yes | Within 24 hours: (1) 36/108 (2) 10/108 |
Only reported as number of events occurring rather than number of participants with specific AEs | No drug‐related serious AEs | (1) 6/117 (2) 1/109 |
10 participants treated only 1 attack and were excluded from the cross‐over efficacy analyses. |
| Mathew 1992 | (1) Sumatriptan 1 mg, n = 30 (2) Sumatriptan 2 mg, n = 30 (3) Sumatriptan 3 mg, n = 30 (4) Sumatriptan 4 mg, n = 30 (5) Sumatriptan 6 mg, n = 30 (6) Sumatriptan 8 mg, n = 30 (7) Placebo, n = 62 |
No | Within 24 hours: (1) 19/30 (2) 20/30 (3) 24/30 (4) 25/30 (5) 26/30 (6) 29/30 (7) 34/62 |
Most common events: Flushing: (1) 0/30; (2) 1/30; (3) 2/30; (4) 1/30; (5) 7/30; (6) 3/30; (7) 1/62 Throat symptoms: (1) 0/30; (2) 1/30; (3) 2/30; (4) 1/30; (5) 1/30; (6) 1/30; (7) 0/62 Nausea and/or vomiting: (1) 3/30; (2) 4/30; (3) 1/30; (4) 6/30; (5) 6/30; (6) 5/30; (7) 10/62 Injection‐site reaction: (1) 15/30; (2) 17/30; (3) 19/30; (4) 20/30; (5) 18/30; (6) 25/30; (7) 21/62 Pressure sensation: (1) 0/30; (2) 1/30; (3) 2/30; (4) 3/30; (5) 2/30; (6) 1/30; (7) 0/62 Feeling of heaviness: (1) 0/30; (2) 0/30; (3) 1/30; (4) 1/30; (5) 1/30; (6) 4/30; (7) 1/62 Chest symptoms: (1) 2/30; (2) 0/30; (3) 1/30; (4) 1/30; (5) 0/30; (6) 3/30; (7) 1/62 Disorder of mouth/tongue: (1) 2/30; (2) 1/30; (3) 1/30; (4) 1/30; (5) 2/30; (6) 2/30; (7) 1/62 Weakness: (1) 0/30; (2) 0/30; (3) 1/30; (4) 0/30; (5) 3/30; (6) 2/30; (7) 0/62 Neck pain/stiffness: (1) 1/30; (2) 1/30; (3) 2/30; (4) 1/30; (5) 3/30; (6) 1/30; (7) 1/62 Feeling of tightness: (1) 0/30; (2) 0/30; (3) 1/30; (4) 0/30; (5) 2/30; (6) 3/30; (7) 0/62 Myalgia: (1) 0/30; (2) 0/30; (3) 0/30; (4) 0/30; (5) 0/30; (6) 3/30; (7) 0/62 Migraine: (1) 1/30; (2) 1/30; (3) 0/30; (4) 4/30; (5) 2/30; (6) 1/30; (7) 0/62 Drowsiness/sedation: (1) 2/30; (2) 0/30; (3) 0/30; (4) 2/30; (5) 0/30; (6) 0/30; (7) 0/62 Dizziness/vertigo: (1) 1/30; (2) 3/30; (3) 2/30; (4) 2/30; (5) 3/30; (6) 2/30; (7) 6/62 Malaise/fatigue: (1) 0/30; (2) 1/30; (3) 1/30; (4) 2/30; (5) 1/30; (6) 0/30; (7) 1/62 Feeling strange: (1) 1/30; (2) 1/30; (3) 2/30; (4) 0/30; (5) 0/30; (6) 1/30; (7) 0/62 Burning sensation: (1) 1/30; (2) 1/30; (3) 2/30; (4) 2/30; (5) 2/30; (6) 2/30; (7) 0/62 Numbness: (1) 1/30; (2) 1/30; (3) 0/30; (4) 1/30; (5) 3/30; (6) 1/30; (7) 2/62 Tingling: (1) 1/30; (2) 1/30; (3) 2/30; (4) 3/30; (5) 7/30; (6) 4/30; (7) 3/62 Cold sensation: (1) 0/30; (2) 0/30; (3) 0/30; (4) 2/30; (5) 1/30; (6) 2/30; (7) 1/62 Warm/hot sensation: (1) 0/30; (2) 3/30; (3) 2/30; (4) 2/30; (5) 5/30; (6) 3/30; (7) 2/62 Headache: (1) 0/30; (2) 0/30; (3) 1/30; (4) 0/30; (5) 1/30; (6) 4/30; (7) 0/62 Sweating: (1) 0/30; (2) 0/30; (3) 1/30; (4) 0/30; (5) 3/30; (6) 1/30; (7) 0/62 |
No data | No data | No data |
| Mushet 1996 | Study 1 (1) Sumatriptan 6 mg, n = 40 (2) Placebo, n = 39 Study 2 (1) Sumatriptan 6 mg, n = 40 (2) Placebo, n = 39 |
No | No data | Occurring in ≥ 5% of participants in any treatment group within 24 hours (results from study 1 and study 2 pooled): Injection‐site reaction: (1) 27/79; (2) 14/79 Nausea/vomiting: (1) 10/79; (2) 11/79 Migraine: (1) 10/79; (2) 7/79 Tingling: (1) 8/79; (2) 4/79 Warm/hot sensation: (1) 5/79; (2) 3/79 Disease of nasal cavity: (1) 3/79; (2) 4/79 Disorder of mouth/tongue: (1) 5/79; (2) 2/79 Flushing: (1) 4/79; (2) 2/79 Malaise/fatigue: (1) 3/79; (2) 3/79 Dizziness/vertigo: (1) 3/79; (2) 2/79 Chest symptoms: (1) 5/79; (2) 0/79 Feeling of heaviness: (1) 5/79; (2) 0/79 Headache: (1) 4/79; (2) 1/79 Joint symptoms: (1) 3/79; (2) 0/79 Weakness: (1) 3/79; (2) 0/79 Burning sensation: (1) 4/79; (2) 0/79 Neck pain/stiffness: (1) 2/79; (2) 1/79 Muscle cramps: (1) 1/79; (2) 2/79 Numbness: (1) 2/79; (2) 0/79 Feeling of tightness: (1) 2/79; (2) 0/79 |
(1) 0/79 (2) 0/79 |
(1) 0/79 (2) 0/79 |
No participant discontinued the study |
| Pfaffenrath 1991 | (1) Sumatriptan 6 mg, n = 155 (2) Placebo, n = 80 |
Yes | Within 24 hours: (1) 60/155 (2) 15/80 |
Most commonly reported after a single dose only: Flushing: (1) 7/107; (2) 0/20 Nausea/vomiting: (1) 7/107; (2) 0/20 Injection‐site reaction: (1) 11/107; (2) 0/20 Neck pain/stiffness: (1) 5/107; (2) 0/20 Migraine: (1) 0/107; (2) 1/20 Dizziness/vertigo: (1) 7/107; (2) 0/20 Malaise/fatigue: (1) 4/107; (2) 1/20 Numbness: (1) 3/107; (2) 0/20 Feeling of heaviness: (1) 3/107; (2) 1/20 |
No data | (1) 3/155 (2) 0/80 |
13 participants were excluded from the efficacy analyses (7 from sumatriptan group and 6 from placebo group): 2 did not provide diary card data 11 failed to use the auto‐injector properly |
| Russell 1994 | (1) Sumatriptan 6 mg, n = 209 (2) Placebo, n = 209 |
Yes | Within 24 hours (after 1 dose only): (1) 35/102 (2) 1/41 |
Most frequent events after single dose only: Injection‐site reaction: (1) 3/102; (2) 0/41 Tachycardia: (1) 4/102; (2) 0/41 Chest symptoms: (1) 2/102; (2) 0/41 Dizziness/vertigo: (1) 7/102; (2) 0/41 Nausea and/or vomiting: (1) 2/102; (2) 0/41 Headache: (1) 2/102; (2) 0/41 Paraesthesia: (1) 7/102; (2) 0/41 Pressure sensation: (1) 4/102; (2) 0/41 Dyspnoea: (1) 2/102; (2) 0/41 Discomfort: (1) 2/102; (2) 0/41 |
(1) 1/224 (2) 0/220 |
(1) 3/224 (2) 0/220 |
21 participants were excluded from the cross‐over efficacy analyses: 16 treated only 1 attack (10 with sumatriptan and 6 with placebo) 5 had missing diary data for 1 or both attacks |
| S2BL99 | (1) Sumatriptan 6 mg, n = 125 (2) Oral ASA 1000 mg + MCP 10 mg, n = 130 |
Yes | Within 24 hours: (1) 59/125 (2) 27/130 |
Malaise/fatigue: (1) 12/125; (2) 4/130 Throat symptoms: (1) 7/125; (2) 0/130 Tingling: (1) 7/125; (2) 0/130 Dizziness/vertigo: (1) 6/125; (2) 1/130 Nausea and/or vomiting: (1) 6/125; (2) 9/130 Burning sensation: (1) 5/125; (2) 0/130 Flushing: (1) 5/125; (2) 0/130 Injection‐site reaction: (1) 5/125; (2) 1/130 Warm/hot sensation: (1) 5/125; (2) 0/130 Pruritis: (1) 4/125; (2) 1/130 Chest symptoms: (1) 4/125; (2) 0/130 Neck pain/stiffness: (1) 4/125; (2) 0/130 Paraesthesia: (1) 4/125; (2) 0/130 Drowsiness/sedation: (1) 2/125; (2) 6/130 Disease of nasal cavity/sinuses: (1) 2/125; (2) 1/130 Dyspnoea: (1) 2/125; (2) 1/130 Abdominal discomfort: (1) 1/125; (2) 3/130 Diarrhoea: (1) 1/125; (2) 2/130 Palpitations: (1) 1/125; (2) 1/130 Gastric symptoms: (1) 1/125; (2) 1/130 Disorder of mouth/tongue: (1) 1/125; (2) 1/130 Feeling of heaviness: (1) 0/125; (2) 1/130 |
(1) 0/125 (2) 1/130 |
(1) 6/125 (2) 1/130 |
Withdrawals due to lack of efficacy: (1) 2/125* (2) 0/130 Other withdrawals: (1) 2/125 (2) 7/130 *One subject withdrew due to both AE and lack of efficacy and is counted in both groups |
| S2BM03 | (1) Sumatriptan 6 mg (+ placebo at 4 h), n = 106 (90 for cross‐over efficacy analysis, of which 87 had moderate or severe baseline pain intensity) (2) Placebo (+ sumatriptan 6 mg at 4 h), n = 106 (90 for cross‐over efficacy analysis, of which 81 had moderate or severe baseline pain intensity) |
Yes | No useable data | No useable data | (1) 0/106 (2) 0/106 |
(1) 0/106 (2) 0/106 |
Withdrawn for other reasons after randomisation (some before taking study medication): (1) 17/106 (2) 19/106 |
| S2BS78 | (1) Sumatriptan 6 mg, n = 236 (2) Placebo, n = 117 |
Yes | No useable data | No useable data | (1) 0/236 (2) 0/117 |
(1) 15/236 (2) 4/117 |
Withdrawn for other reasons after randomisation (some before taking study medication): (1) 28/249 (2) 14/122 |
| Sang 2004 | (1) Sumatriptan 6 mg, n = 15 (2) Intravenous LY293558 1.2 mg/kg, n = 13 (3) Placebo, n = 16 |
No | Within 24 hours: (1) 8/15 (2) 2/13 (3) 5/16 |
Reported by > 10% of participants: Chest/throat symptoms: (1) 2/15; (2) 0/13; (3) 0/16 Disorientation: (1) 4/15; (2) 1/13; (3) 1/16 Dizziness: (1) 4/15; (2) 2/13; (3) 2/16 Heaviness/tingling: (1) 5/15; (2) 0/13; (3) 0/16 Sedation/drowsiness: (1) 5/15; (2) 2/13; (3) 4/16 Visual symptoms: (1) 4/15; (2) 1/13; (3) 1/16 Warmth: (1) 5/15; (2) 1/13; (3) 1/16 |
No data | No data | 1 participant randomised to LY293558 withdrew before receiving treatment |
| Schulman 2000 | (1) Sumatriptan 6 mg, n = 79 (2) Placebo, n = 40 |
No | Drug‐related within 24 hours: (1) 15/79 (2) 3/40 |
No data | (1) 0/79 (2) 0/40 |
(1) 1/79 (2) 0/40 |
3 participants were excluded from the efficacy analyses (all in sumatriptan group) due to incomplete diary data |
| SUM40286 | (1) Sumatriptan 6 mg, n = 146 (145 for efficacy) (2) Placebo, n = 153 (152 for efficacy) |
Yes | Up to final visit: (1) 36/146 (2) 21/153 |
Nausea: (1) 9/146; (2) 3/153 Other pressure/tightness: (1) 9/146; (2) 0/153 Injection‐site reaction: (1) 8/146; (2) 3/153 Dizziness: (1) 6/146; (2) 0/153 Chest symptoms: (1) 5/146; (2) 0/153 Temperature sensation: (1) 5/146; (2) 2/153 Paraesthesia: (1) 3/146; (2) 2/153 Migraine: (1) 3/146; (2) 1/153 Burning/stinging sensation: (1) 2/146; (2) 1/153 Headache: (1) 2/146; (2) 1/153 Disturbance of sense of taste: (1) 0/146; (2) 1/153 Malaise and fatigue: (1) 0/146; (2) 1/153 Nasal inflammation: (1) 0/146; (2) 1/153 Sinusitis: (1) 0/146; (2) 1/153 Temperature regulation disturbances: (1) 0/146; (2) 1/153 Throat and tonsil discomfort and pain: (1) 0/146; (2) 1/153 |
(1) 0/146 (2) 0/153 |
(1) 0/146 (2) 0/153 |
Withdrawn for other reasons after randomisation (some before taking study medication): (1) 29/175 (2) 30/182 |
| SUM40287 | (1) Sumatriptan 6 mg, n = 149 (148 for efficacy) (2) Placebo, n = 139 |
Yes | Up to final visit: (1) 45/149 (2) 13/139 |
Injection‐site reaction: (1) 7/149; (2) 2/139 Temperature sensation: (1) 7/149; (2) 0/139 Nausea: (1) 6/149; (2) 3/139 Paraesthesia: (1) 6/149; (2) 1/139 Dizziness: (1) 5/149; (2) 3/139 Chest symptoms: (1) 4/149; (2) 2/139 Malaise and fatigue: (1) 2/149; (2) 0/139 Breathing disorder: (1) 1/149; (2) 0/139 Headache: (1) 1/149; (2) 0/139 Nasal signs and symptoms: (1) 1/149; (2) 0/139 Other pressure/tightness: (1) 1/149; (2) 0/139 Sweating: (1) 1/149; (2) 2/139 Tachycardia: (1) 1/149; (2) 1/139 Temperature regulation disturbances: (1) 1/149; (2) 0/139 Vomiting: (1) 1/149; (2) 2/139 Disturbance of sense of taste: (1) 0/149; (2) 1/139 Drowsiness: (1) 0/149; (2) 1/139 Somnolence: (1) 0/149; (2) 1/139 |
(1) 0/149 (2) 0/139 |
(1) 0/149 (2) 0/139 |
Withdrawn for other reasons after randomisation (some before taking study medication): (1) 28/177 (2) 36/174 |
| Thomson 1993 | (1) Sumatriptan 4 mg, n = 28 (2) Placebo, n = 23 |
No | Within 24 hours: (1) 23/28 (2) 4/23 |
No data | (1) 0/28 (2) 0/23 |
No data | 1 participant was excluded from efficacy analyses because of protocol violation (use of ergotamine within 24 hours) |
| Touchon 1996 | (1) Sumatriptan 6 mg, n = 278 (2) DHE nasal spray 1 mg, n = 277 |
Yes | Within 24 hours: (1) 120/278 (2) 62/277 |
No data | No data | (1) 3/278 (2) 1/277 |
12 participants withdrawn after treating first attack (including 4 adverse event withdrawals) 11 participants failed to treat a second attack, therefore 266 participants evaluable for cross‐over efficacy analyses. |
| Visser 1992 | (1) Sumatriptan 1 mg, n = 170 (2) Sumatriptan 2 mg, n = 171 (3) Sumatriptan 3 mg, n = 172 (4) Placebo, n = 172 |
Yes | Only pooled results for all 3 doses of sumatriptan versus placebo given | Only pooled results for all 3 doses of sumatriptan versus placebo given | (1) 0/170 (2) 0/171 (3) 0/172 (4) 0/172 |
(1) 0/170 (2) 0/171 (3) 0/172 (4) 0/172 |
No data |
| Wendt 2006 | (1) Sumatriptan 4 mg, n = 384 (2) Placebo, n = 193 |
No | Within 24 hours: (1) 265/384 (2) 75/193 |
Occurring in ≥ 1% of participants in either treatment group: Injection‐site reaction: (1) 165/384; (2) 28/193 Tingling: (1) 45/384; (2) 6/193 Dizziness or vertigo: (1) 40/384; (2) 10/193 Warm or hot sensation: (1) 30/384; (2) 4/193 Nausea and/or vomiting: (1) 28/384; (2) 15/193 Pressure sensation: (1) 22/384; (2) 2/193 Burning sensation: (1) 20/384; (2) 1/193 Chest symptoms: (1) 20/384; (2) 2/193 Feeling of heaviness: (1) 20/384; (2) 1/193 Disorder of mouth or tongue: (1) 17/384; (2) 2/193 Numbness: (1) 12/384; (2) 5/193 Drowsiness or sedation: (1) 11/384; (2) 4/193 Flushing: (1) 10/384; (2) 7/193 Malaise/fatigue: (1) 9/384; (2) 3/193 Disturbance of hearing: (1) 8/384; (2) 0/193 Feeling strange: (1) 7/384; (2) 3/193 Neck pain or stiffness: (1) 7/384; (2) 1/193 Cold sensation: (1) 7/384; (2) 0/193 Sweating: (1) 6/384; (2) 1/193 Nasal or sinus discomfort: (1) 5/384; (2) 2/193 Tight feeling in head: (1) 5/384; (2) 0/193 Weakness: (1) 5/384; (2) 3/193 Anxiety: (1) 4/384; (2) 0/193 Throat symptoms: (1) 4/384; (2) 0/193 |
(1) 1/384 (2) 5/193 |
No data | No data |
| Winner 1996 | (1) Sumatriptan 6 mg, n = 158 (2) Subcutaneous dihydroergotamine (DHE) mesylate 1 mg, n = 152 |
Yes | Only number of events reported rather than number of participants with event | Clinically relevant AEs occurring within 24 hours: Nausea: (1) 9/158; (2) 24/152 Vomiting: (1) 6/158; (2) 10/152 Chest pain: (1) 9/158; (2) 1/152 |
(1) 0/158 (2) 0/152 |
(1) 0/158 (2) 2/152 |
No data |
| Winner 2006 | Study 1 (1) Sumatriptan 6 mg, n = 146 (2) Placebo, n = 153 Study 2 (1) Sumatriptan 6 mg, n = 149 (2) Placebo, n = 139 |
Yes | Drug‐related (results from study 1 and study 2 pooled): (1) 71/295 (2) 18/292 |
Most commonly reported: Nausea: Study 1 (1) 9/146; (2) 3/153 Study 2 (1) 6/149; (2) 3/139 Injection‐site reaction: Study 1 (1) 7/146; (2) 3/153 Study 2 (1) 7/149; (2) 1/139 |
Study 1 (1) 0/146 (2) 0/153 Study 2 (1) 0/149 (2) 0/139 |
Study 1 (1) 0/146 (2) 0/153 Study 2 (1) 0/149 (2) 0/139 |
5 participants excluded from efficacy analysis: 3 did not return evaluable data 2 did not have sufficient baseline pain intensity (1 from sumatriptan and one from placebo group in study 1) |
Appendix 9. Breakdown of individual adverse event groups
We used the following groupings of individual adverse events in all four reviews of sumatriptan whenever it was possible to combine studies for analysis (all routes of administration except rectal).
Malaise/fatigue/asthenia:
Malaise/fatigue
Fatigue
Malaise and fatigue
Asthenia/fatigue
Fatigue/weakness
Asthenia
Weakness
Dizziness/vertigo:
Dizziness/vertigo
Dizziness
Dizziness (excl. vertigo)
Dizziness (not vertigo)
Nausea/vomiting:
Nausea/vomiting
Nausea
Vomiting
Nausea and vomiting
Disorder of mouth/disturbance of taste:
Disorder of mouth/tongue
Mouth disorder
Dry mouth
Disturbance of taste
Bad taste
Drug taste
Chest pain/symptoms:
Chest pressure/heaviness
Chest tightness
Chest discomfort
Chest pain
Chest symptoms
Constriction of throat/chest pain
Tightness of throat
Heat sensations/flushing:
Warm/hot sensation
Flushing
Vasodilation
Heat flashes
Warm sensation
Temperature sensations
Hot flush
Burning sensation
Palpitations/tachycardia:
Palpitations
Tachycardia
Diarrhoea:
Diarrhoea
Feeling of tightness/heaviness:
Feeling of heaviness
Heaviness other than chest or neck
Feeling of heaviness in head
Heaviness/pressure sensation
Heaviness in lower limbs
Heaviness, regional
Head pressure
Tightness
Other pressure/tightness
Sweating:
Sweating
Abdominal pain/discomfort/dyspepsia:
Abdominal discomfort
Abdominal pain
Abdominal pain or cramps
Dyspepsia
Gastric symptoms
Gastroesophageal reflux
Paraesthesia/numbness:
Paraesthesia
Tingling
Numbness/paraesthesia/tingling
Numbness
Headache:
Headache
Drowsiness/somnolence:
Drowsiness/sedation
Somnolence
Sleepiness
Drowsiness
Anxiety:
Anxiety
Neck/back pain:
Neck pain/stiffness
Neck pain
Back or neck pain
Back pain
Disorder of nasal cavity/sinuses:
Disorder of nasal cavity/sinuses
Nasal discomfort
Nasal stuffiness
Wet nostrils
Throat symptoms
Throat symptoms
Throat discomfort
Injection‐site reaction:
Injection‐site reaction
Application site reaction
Appendix 10. L'Abbé plots for sumatriptan 6 mg versus placebo
L'Abbé plots for sumatriptan 6 mg versus placebo for the outcomes pain‐free at two hours (Figure 32), headache relief at one hour (Figure 33), and headache relief at two hours (Figure 34) show consistency in response across studies for these outcomes.
6.
L'Abbé plot showing results for sumatriptan 6 mg versus placebo for pain‐free at two hours. Each circle represents a different study; size of circle is proportional to size of study; diagonal is line of equivalence
7.
L'Abbé plot showing results for sumatriptan 6 mg versus placebo for headache relief at one hour. Each circle represents a different study; size of circle is proportional to size of study (with the exception of two in which publications only reported the pooled results of two individual studies); diagonal is line of equivalence
8.
L'Abbé plot showing results for sumatriptan 6 mg versus placebo for headache relief at two hours. Each circle represents a different study; size of circle is proportional to size of study; diagonal is line of equivalence
Data and analyses
Comparison 1. Subcutaneous sumatriptan 4 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain‐free at 2 h | 2 | 664 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.82 [3.24, 7.17] |
| 2 Pain‐free at 1 h | 2 | 664 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.66 [2.83, 7.67] |
| 3 Headache relief at 1 h | 2 | 664 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.55 [2.02, 3.21] |
| 4 Headache relief at 2 h | 2 | 664 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.12 [2.43, 4.01] |
| 5 Any adverse event within 24 h | 3 | 720 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.56, 2.16] |
Comparison 2. Subcutaneous sumatriptan 6 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain‐free at 2 h | 11 | 2522 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.85 [3.32, 4.46] |
| 2 Pain‐free at 1 h | 14 | 3592 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.55 [4.55, 6.77] |
| 3 Headache relief at 1 h | 21 | 5177 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [2.51, 2.93] |
| 4 Headache relief at 2 h | 12 | 2738 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.50 [2.29, 2.73] |
| 5 24 h sustained pain‐free | 2 | 752 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.18 [1.61, 2.95] |
| 6 Use of rescue medication | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Up to 24 h after initial dosing | 5 | 987 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.45, 0.60] |
| 6.2 Up to 2 h after initial dosing | 4 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.27, 0.44] |
| 7 Relief of associated symptoms | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Relief of nausea at 2 h | 4 | 667 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.22 [1.87, 2.64] |
| 7.2 Relief of photophobia at 2 h | 2 | 631 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [1.59, 2.24] |
| 8 Relief of functional disability | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Any functional disability at baseline to none at 2 hours | 2 | 750 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.40 [2.66, 4.35] |
| 8.2 Moderate or severe functional disability to mild or none at 1 hour | 3 | 1328 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.21 [2.68, 3.84] |
| 9 Any adverse event within 24 h | 9 | 1342 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.08 [1.75, 2.47] |
| 10 Any adverse event withdrawal | 12 | 3287 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [0.90, 4.96] |
| 11 Pain‐free at 2 h ‐ effect of size | 11 | 2522 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.85 [3.32, 4.46] |
| 11.1 Studies containing at least 50 participants in each treatment arm | 6 | 1976 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.57 [3.03, 4.20] |
| 11.2 Studies containing one or more treatment arms with fewer than 50 participants | 5 | 546 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.29 [3.69, 7.58] |
| 12 Pain‐free at 1 h ‐ effect of size | 14 | 3592 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.55 [4.55, 6.77] |
| 12.1 Studies containing at least 50 participants in each treatment arm | 8 | 2985 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.53 [4.45, 6.88] |
| 12.2 Studies containing one or more treatment arms with fewer than 50 participants | 6 | 607 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.64 [3.42, 9.29] |
| 13 Headache relief at 1 h ‐ effect of size | 21 | 5177 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [2.51, 2.93] |
| 13.1 Studies containing at least 50 participants in each treatment arm | 10 | 4040 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.73 [2.50, 2.99] |
| 13.2 Studies containing one or more treatment arms with fewer than 50 participants | 11 | 1137 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.65 [2.26, 3.10] |
| 14 Headache relief at 2 h ‐ effect of size | 12 | 2738 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.50 [2.29, 2.73] |
| 14.1 Studies containing at least 50 participants in each treatment arm | 7 | 2192 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.45 [2.22, 2.70] |
| 14.2 Studies containing one or more treatment arms with fewer than 50 participants | 5 | 546 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.74 [2.24, 3.36] |
| 15 Pain‐free at 1 h ‐ effect of missing data | 14 | 3592 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.55 [4.55, 6.77] |
| 15.1 Studies with no missing data | 12 | 3208 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.01 [4.09, 6.14] |
| 15.2 Studies with missing data | 2 | 384 | Risk Ratio (M‐H, Fixed, 95% CI) | 35.63 [8.87, 143.18] |
| 16 Headache relief at 1 hour ‐ effect of missing data | 21 | 5177 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [2.51, 2.93] |
| 16.1 Studies with no missing data | 19 | 4793 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.56 [2.36, 2.77] |
| 16.2 Studies with missing data | 2 | 384 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.64 [5.66, 16.42] |
| 17 Headache relief at 2 hours ‐ effect of missing data | 12 | 2738 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.50 [2.29, 2.73] |
| 17.1 Studies with no missing data | 10 | 2354 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.26 [2.07, 2.47] |
| 17.2 Studies with missing data | 2 | 384 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.39 [4.78, 11.41] |
2.10. Analysis.
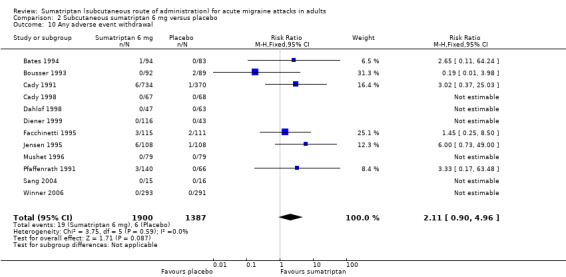
Comparison 2 Subcutaneous sumatriptan 6 mg versus placebo, Outcome 10 Any adverse event withdrawal.
Comparison 3. Subcutaneous sumatriptan 8 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain‐free at 1 h | 2 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.19 [3.86, 13.41] |
| 2 Headache relief at 1 h | 3 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.58 [2.71, 4.72] |
Comparison 4. Subcutaneous sumatriptan 6 mg (+ optional 6 mg) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain‐free at 2 h | 3 | 388 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.59 [2.85, 7.39] |
| 2 Headache relief at 2 h | 5 | 1728 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.39 [2.13, 2.69] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akpunonu 1995.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered when migraine headache pain was of moderate or severe intensity Assessments by stopwatch and at discharge from emergency department (time not reported and may vary between participants) Rescue medication (excluding ergot derivatives) available after 90 minutes if headache relief not achieved Each participant provided with an open‐label 100 mg sumatriptan tablet to treat recurrence over the 24 h period after discharge |
|
| Participants | Aged 18 years or older, meeting IHS criteria for migraine (1988) with aura. At least 1‐year history of migraine. Participants with a frequency of tension headache of at least 15 days per month were excluded No concurrent use of monoamine oxidase inhibitors, lithium, or selective 5‐HT reuptake inhibitors No use of ergotamine within 24 h of study drug administration N = 136 Breakdown of participants by gender not reported Mean age not reported 100% with aura |
|
| Interventions | Sumatriptan 6 mg, n = 88 Placebo, n = 48 |
|
| Outcomes | Adverse events | |
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | High risk | Treatment group 50 to 200 participants, placebo group < 50 participants |
Bates 1994.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered at onset of migraine aura Assessments at 0.5, 1, 1.5, 2, 4, 6, 12, and 24 h after dosing Second unblinded dose of sumatriptan 6 mg available after 2 h for inadequate relief Rescue medication available 2 h after second dose of study medication |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with aura. At least 6‐month history of migraine (untreated severity ≥ moderate) and at least 50% of attacks with aura. Excluded participants with previous use of subcutaneous sumatriptan N = 177 (171 for efficacy, 82 with moderate or severe baseline pain intensity) M 46, F 125 (73%) Mean age 40 years All treated attacks with aura |
|
| Interventions | Sumatriptan 6 mg, n = 90 (88 for efficacy, 47 with moderate or severe baseline pain intensity) Placebo, n = 87 (83 for efficacy, 35 with moderate or severe baseline pain intensity) |
|
| Outcomes | Headache relief (at 1 h) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Matching placebo |
| Study size | High risk | Treatment groups < 50 participants |
Bousser 1993.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, cross‐over 2 consecutive early‐morning attacks treated when migraine headache pain was of moderate or severe intensity Single dose to treat each of 2 successive attacks with recommended second dose of study medication after 1 h for inadequate relief Assessments at 1, 2, 4, and 24 h after dosing Rescue medication available 2 h after initial dosing, provided it did not contain ergotamine |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 6‐month history of migraine (untreated severity ≥ moderate) with an average of 2 to 6 attacks per month, of which at least 2 were early‐morning migraine attacks. No ergot‐containing preparations were allowed within 24 h of taking study drugs N = 96 M 17, F 79 (82%) Mean age 41 years Proportion with/without aura not reported |
|
| Interventions | Sumatriptan 6 mg, n = 49 (41 for 1st attack efficacy) Placebo, n = 47 (40 for 1st attack efficacy) |
|
| Outcomes | Headache relief (at 1 h) and 2 h (1 h after optional 2nd dose) Pain‐free (at 1 h) and 2 h (1 h after optional 2nd dose) Presence of nausea and vomiting at 1 h Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation code |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study drug and placebo provided in identical syringes |
| Study size | High risk | Treatment groups < 50 participants |
Cady 1991.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group Single dose to treat single attack, with the option of a second randomised dose of study medication or placebo if pain relief was inadequate at 1 h Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 10, 20, 30, 40, 50, 60, 90, and 120 minutes after dosing Rescue medication available at the discretion of the investigator if migraine persisted 1 h after second dose of study medication 2 separate identical trials |
|
| Participants | Aged 18 years or over, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate). Participants excluded if previously treated with sumatriptan. Long‐term prophylactic medications for migraine allowed. No opioids or ergotamine within 24 h, or simple analgesics within 6 h of taking study medication. Study 1 N = 574 M 73, F 501 (87%) Mean age 40 years Proportion with/without aura not reported Study 2 N = 530 M 53, F 477 (90%) Mean age 39 years Proportion with/without aura not reported |
|
| Interventions | Study 1 Sumatriptan 6 mg, n = 384 Placebo, n = 190 Study 2 Sumatriptan 6 mg, n = 350 Placebo, n = 180 |
|
| Outcomes | All outcomes reported as pooled results from the 2 studies (Study 1 and Study 2) Headache relief (at 1 h) and 2 h (1 h after optional 2nd dose) Pain‐free (at 1 h) Improvement in nausea and photophobia at 1 h Improvement in functional disability at 1 h Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Allocation based on chronological order that patients presented for treatment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | Low risk | Treatment groups > 200 participants |
Cady 1993.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, cross‐over. Single dose to treat each of 4 consecutive attacks (3 with sumatriptan, 1 with placebo). Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 0.5, 1, and 1.5 h after dosing Rescue medication available after 1.5 h |
|
| Participants | Aged 18 years or over, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate). No ergotamine or analgesics containing opioid derivatives within 24 h, or simple analgesics or antiemetics within 6 h of taking study medication Each treatment separated by a pain‐free interval of at least 24 h N = 170 (of which 120 treated all 4 attacks) M 15, F 155 (91%) Mean age 41 years Proportion with/without aura not reported |
|
| Interventions | Sumatriptan 6 mg, n = 166 (128 treating first attack with moderate or severe baseline pain intensity) Placebo, n = 144 (42 treating first attack with moderate or severe baseline pain intensity) |
|
| Outcomes | Headache relief (at 1 h) Pain‐free (at 1 h) 24 h sustained headache relief 24 h sustained pain‐free Improvement in nausea, vomiting, photophobia, and phonophobia at 1 h Improvement in functional disability at 1 h Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo injections designed to match the active dose |
| Study size | High risk | Treatment group 50 to 200 participants, placebo group < 50 participants |
Cady 1998.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered when migraine headache of moderate or severe intensity occurred within the first 4 h of a minimum 8 h work shift Assessments at 1 and 2 h after dosing Rescue medication (with the exception of ergotamine‐containing medications or sumatriptan) available after 2 h for intolerable pain Second dose of study medication available to treat recurrence in the workplace, provided no use of rescue medication had occurred |
|
| Participants | Aged 18 years or over, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate) with an average of 1 to 6 attacks per month. Participants had to have treated at last 1 disabling migraine in the workplace in the past 60 days, and had to be working 8‐hour (minimum) shifts at their jobs No monoamine oxidase inhibitors within 2 weeks of screening. No ergotamine‐containing medications or sumatriptan within 24 h, and no analgesics, antiemetics, or other acute migraine medications within 6 h of taking study medication. Participants were excluded if they had previously used sumatriptan (any formulation) N = 135 (132 for efficacy) M 20, F 112 (85%) Mean age 40 years Without aura 69% |
|
| Interventions | Sumatriptan 6 mg, n = 67 Placebo, n = 68 (65 for efficacy) |
|
| Outcomes | Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Matching placebo |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
Dahlof 1992.
| Methods | Single‐centre, randomised, double‐blind, placebo‐controlled, within‐patient cross‐over. Each participant treated 2 successive attacks with a single dose of one or other study medication. Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 0.5, 1, 1.5, and 2 h after dosing Rescue medication (not ergotamine) was available after 2 h for inadequate relief of symptoms |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate) with an average of 1 to 6 attacks per month. Use of migraine prophylactic therapy was stopped at least 2 weeks before receipt of study medication. No ergotamine‐containing preparations within 24 h, and no analgesics within 6 h of taking study medication. Minimum of 48 h between treated attacks N = 27 M 5, F 22 (81%) Mean age 45 years Proportion with/without aura not reported |
|
| Interventions | Sumatriptan 8 mg, n = 27 Placebo, n = 27 |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 2 h) Use of rescue medication Presence of functional disability (at 1 and 2 h) |
|
| Notes | Oxford Quality Score: R1, DB2, W0. Total = 3. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Matching placebo |
| Study size | High risk | Treatment groups < 50 participants |
Dahlof 1998.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered when migraine headache pain was of moderate to severe intensity Assessments at 10, 20, 30, 60, 90, 120, 180, and 240 minutes after dosing Rescue medication (excluding ergotamine‐containing therapy) was available after 4 h for inadequate relief of symptoms |
|
| Participants | Aged 18 to 55 years, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate) with an average of 1 to 6 attacks per month. Participants were excluded if they had previously received subcutaneous sumatriptan Migraine prophylactic therapy stopped at least 2 weeks before the administration of study treatment No ergotamine‐containing preparations within 24 h, or analgesics within 6 h of receiving study medication N = 335 M 47, F 288 (86%) Mean age 38 years Without aura 89% |
|
| Interventions | Sumatriptan 6 mg, n = 47 Naratriptan 0.5 mg, n = 60 Naratriptan 1 mg, n = 55 Naratriptan 2.5 mg, n = 42 Naratriptan 5 mg, n = 34 Naratriptan 10 mg, n = 34 Placebo, n = 63 |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 2 h) Improvement in nausea, vomiting, and photo/phonophobia at 2 h Presence of functional disability at 2 h Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | High risk | Some treatment groups 50 to 200 participants, others < 50 participants |
Diener 1999.
| Methods | Multicentre, randomised, double‐blind, double‐dummy, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered when migraine headache pain was of moderate or severe intensity Assessment at 0.5, 1, 1.5, and 2 h after dosing Rescue medication available after 2 h |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate) with an average of 2 to 6 attacks per month. No analgesics or migraine drugs within 24 h of study medication administration. No use of compound analgesics, sumatriptan, ergotamine tartrate, DHE, codeine, or barbiturates for more than 10 days per month prior to screening. N = 278 (275 for efficacy) M 55, F 220 (80%) Mean age 41 years Without aura 67% |
|
| Interventions | Sumatriptan 6 mg, n = 114 Intravenous acetylsalicylic acid lysinate 1.8 g, n = 119 Placebo, n = 42 |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 2 h) Improvement in nausea, vomiting, photophobia, and phonophobia at 2 h Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy |
| Study size | High risk | Treatment groups 50 to 200 participants, placebo group < 50 participants |
Diener 2001.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered when migraine headache pain was of moderate or severe intensity, after any aura symptoms had resolved Assessments at 0.25, 1, and 2 h after dosing Rescue medication (excluding sumatriptan and ergotamine‐derivatives) was available after 2 h if needed |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 6‐month history of migraine (untreated severity ≥ moderate) with an average of 1 to 6 attacks per month. Each treated attack associated with 1 of the following symptoms: nausea, vomiting, photophobia, or phonophobia Participants were excluded if they used acute migraine medication (ergotamine, ergot‐derivatives, sumatriptan, aspirin, or NSAIDs) for more than 10 days per month No long‐term prophylactic migraine therapy with methysergide, tricyclic antidepressants, or monoamine oxidase inhibitors (although prophylactic therapy with flunarizine, pizotifen, or beta‐blockers started before the trial was not a reason for exclusion) N = 924 M 126, F 798 (86%) Mean age 41 years Without aura 86% |
|
| Interventions | Sumatriptan 6 mg, n = 317 Alniditan 1.4 mg, n = 309 Alniditan 1.8 mg, n = 141 Placebo, n = 157 (156 for efficacy) |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 2 h) Use of rescue medication Improvement in functional disability at 1 h Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | Unclear risk | Some treatment groups > 200 participants, others and placebo group 50 to 200 participants |
Facchinetti 1995.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat each of 2 attacks occurring ‐3 to +5 days relative to the first day of menstruation. Assessments at 1, 2, and 24 h after dosing Second dose of study medication available to treat recurrence within 24 h Rescue medication (excluding ergotamine‐containing preparations or sumatriptan) available if relief was inadequate after 2 h |
|
| Participants | Female participants, aged 18 to 50 years, meeting IHS criteria for migraine (1988) without aura. At least 6‐month history of migraine occurring ‐3 to +5 days relative to the first day of menstruation and a history of regular menstrual cycles. N = 226 (169 for first dose efficacy assessment with moderate or severe baseline pain intensity) F 226 Mean age 37 years 3% to 6% of subjects with aura (included in efficacy analyses) |
|
| Interventions | Sumatriptan 6 mg, n = 115 (77 for first dose efficacy with moderate or severe baseline pain intensity) Placebo, n = 111 (92 for first dose efficacy with moderate or severe baseline pain intensity) |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 1 and 2 h) Improvement in nausea and photo/phonophobia at 2 h Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation scheme |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Matching placebo‐filled syringes |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
Ferrari 1991.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 1, 2, and 24 h after dosing Second blinded and re‐randomised dose of study medication available if, after 1 h, the patient was not completely pain‐free Rescue medication (excluding ergotamine and dihydroergotamine) available after 2 h if symptoms were not improved at this time |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate) with a maximal frequency of 6 attacks per month. No prophylaxis for migraine within 2 weeks, ergot‐containing preparations within 24 h, or simple analgesics/NSAIDs within 6 h of taking study medication N = 639 (636 for efficacy) M 118, F 521 (82%) Mean age 40 years Without aura 70% |
|
| Interventions | Sumatriptan 6 mg, n = 423 (422 for efficacy) Sumatriptan 8 mg, n = 110 (109 for efficacy) Placebo, n = 106 (105 for efficacy) |
|
| Outcomes | Headache relief (at 1 h) and 2 h (1 h after optional 2nd dose) Pain‐free (at 1 h) |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation code |
| Allocation concealment (selection bias) | Low risk | Patients were entered in ascending sequential order at each centre |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo was supplied in matching ampoules containing isotonic saline solution |
| Study size | Unclear risk | One treatment group > 200 participants, other treatment and placebo group 50 to 200 participants |
Gross 1994.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat single attack. Assessments at 1 and 2 h after dosing Second dose of study medication available for inadequate relief after 1 h or for recurrence between 1 and 24 h Alternative rescue medication (excluding ergotamine‐containing medications) available 1 h after the second dose of study medication if migraine relief still inadequate |
|
| Participants | Aged 18 to 65, meeting IHS criteria for migraine (1988) with or without aura. At least 6‐month history of migraine (untreated severity ≥ moderate) with an average of 1 to 6 attacks per month. Participants were excluded if they had previously used sumatriptan to treat more than 6 migraine attacks N = 86 M 17, F 69 (82%) Mean age 44 years Without aura 70% |
|
| Interventions | Sumatriptan 6 mg, n = 60 (48 with moderate or severe baseline pain intensity) Placebo, n = 26 (18 with moderate or severe baseline pain intensity) |
|
| Outcomes | Headache relief (at 1 h) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | High risk | Treatment group 50 to 200 participants, placebo group < 50 participants |
Henry 1993.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 1, 2, and 4 h after dosing Second identical dose of study medication available after 1 h if participants had inadequate relief or for recurrence between 2 and 24 h Alternative rescue medication (non‐ergotamine) was available after 2 h for either inadequate relief or recurrence |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura Participants were required to have been treating with oral dihydroergotamine correctly for migraine prophylaxis for at least 1 month, which could be maintained at the same dose schedule for the duration of the study N = 76 M 10, F 66 (87%) Mean age 43 years Proportion with/without aura not reported |
|
| Interventions | Sumatriptan 6 mg, n = 37 Placebo, n = 39 |
|
| Outcomes | Headache relief (at 1 h) and 2 h (1 h after optional 2nd dose) Pain‐free (at 1 h) and 2 h (1 h after optional 2nd dose) Improvement in nausea and vomiting at 1 h Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | High risk | Treatment groups < 50 participants |
Jensen 1995.
| Methods | 2‐phase study Phase one: multicentre, randomised, double‐blind, placebo‐controlled, cross‐over design. Single dose to treat each of 2 successive migraine attacks. Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 0.5, 1, 1.5, and 2 h after initial dosing Second dose of study medication (identical to first dose) available to treat recurrence between 2 and 24 h Rescue medication (except ergotamine) available if initial treatment not effective within 2 h Phase 2: open‐label phase |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. History of 1 to 6 moderate or severe migraine attacks per month. Participants were excluded if they had previous experience with subcutaneous sumatriptan No ergotamine in the 24‐h period before taking study medication or within 6 h afterwards N = 118 treated ≥ 1 attack (108 treated both attacks) M 12, F 106 (90%) Mean age 43 years Proportion with/without aura not reported |
|
| Interventions | Sumatriptan 6 mg, n = 117 attacks Placebo, n = 109 attacks |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 1 h) Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | Unclear risk | Number in each treatment arm for first attack not reported |
Mathew 1992.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 10, 20, 30, 40, and 50 minutes, and 1, 1.5, 2, 2.5, 4, and 4 h after dosing Rescue medication (excluding ergot‐containing drugs) were available at the discretion of the investigator beginning 1 h after dosing. Scores were adjusted for use of rescue medications by carrying the last observation (before rescue) forward. Headache relief could not be achieved if rescue medication was used. |
|
| Participants | Aged 18 or older, meeting IHS criteria for migraine (1988) with or without aura No use of analgesic or ergot‐containing medication within the previous 24 h (or 6 h for simple analgesics) Migraine prophylaxis was allowed N = 242 M 32, F 210 (87%) Mean age 38 years Without aura 80 % |
|
| Interventions | Sumatriptan 1 mg, n = 30 Sumatriptan 2 mg, n = 30 Sumatriptan 3 mg, n = 30 Sumatriptan 4 mg, n = 30 Sumatriptan 6 mg, n = 30 Sumatriptan 8 mg, n = 30 Placebo, n = 62 |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 1 and 2 h) Improvement in nausea and photophobia at 1 h Use of rescue medication Adverse events |
|
| Notes | Oxford Quality Score: R1, DB1, W0. Total = 2. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | High risk | Treatment groups < 50 participants, placebo group 50 to 200 participants |
Mushet 1996.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat single attack. Assessments at 10, 20, 30, 40, 50, 60, 90, and 120 minutes after dosing Rescue medication available after 2 h for participants who had not yet experienced headache relief Identical procedures were followed for each of the 2 studies, Study 1 and Study 2 |
|
| Participants | Aged 18 to 65, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine with an average of 1 to 6 attacks per month during the 2 months before screening. Participants were excluded if they had ever used subcutaneous sumatriptan, although use of oral sumatriptan was not a reason for exclusion Any chronic use of migraine prophylaxis, calcium channel blockers, tricyclic antidepressants, beta‐blockers, and serotoninergics was required to remain unchanged for the duration of the study Study 1 N = 80 M 11, F 69 (86%) Mean age 40 years Without aura 68% Study 2 N = 78 M 10, F 68 (87%) Mean age 39 years Without aura 62% All participants had moderate or severe baseline pain intensity |
|
| Interventions | Study 1 Sumatriptan 6 mg, n = 40 Placebo, n = 39 Study 2 Sumatriptan 6 mg, n = 40 Placebo, n = 39 |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 1 and 2 h) Improvement in nausea, vomiting, photophobia, and phonophobia at 1 h Presence of functional disability (at 1 and 2 h) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Construction of the delivery system prevented the patient or clinician from viewing the syringe contents during the administration procedure |
| Study size | High risk | Treatment groups < 50 participants |
Pfaffenrath 1991.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 1 and 2 h after dosing Second dose of study medication available after 1 h if participants had inadequate relief Alternative rescue medication (excluding ergotamine) was available if relief was still inadequate after 2 h |
|
| Participants | Aged 18 to 65, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine with a maximum of 6 attacks per month. Participants receiving migraine prophylaxis were required to withdraw from prophylactic therapy at least 2 weeks prior to randomisation Ergotamine preparations were not to be used within 24 h of taking test medication N = 235 (216 with moderate or severe baseline pain intensity) M 43, F 192 (82%) Mean age 41 years Without aura 65% |
|
| Interventions | Sumatriptan 6 mg, n = 155 (147 with moderate or severe baseline pain intensity) Placebo, n = 80 (69 with moderate or severe baseline pain intensity) |
|
| Outcomes | Headache relief (at 1 h) and 2 h (1 h after optional 2nd dose) Pain‐free (at 1 h) and 2 h (1 h after optional 2nd dose) Improvement in nausea, vomiting, and photo/phonophobia at 1 h Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation code |
| Allocation concealment (selection bias) | Low risk | Patients were entered in ascending sequential order of patient number at each centre |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo was provided in identical syringes |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
Russell 1994.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, cross‐over design. Single dose to treat each of 2 successive attacks. Assessments at 1 and 2 h after dosing. Second dose of study medication available after 2 h for participants not completely free from headache, or experiencing recurrence of headache within 24 h Rescue medication (non‐ergotamine) was available 1 h after second injection if symptom relief remained inadequate |
|
| Participants | Aged 18 to 65, with GP diagnosed migraine. At least 6‐month history of migraine (untreated severity ≥ moderate) with an average of 1 to 6 attacks per month. Participants were excluded if they had previously used sumatriptan or were currently using migraine prophylactic agents N = 230 (209 treated both attacks) M 20, F 189 (90%) Mean age 44 years Post‐treatment headache diagnosis revealed that ≥ 90% of treated attacks met IHS criteria for migraine (1988) with or without aura. Without aura 65% Approximately 1% of participants had mild baseline pain intensity when study medication was administered |
|
| Interventions | Sumatriptan 6 mg, n = 209 Placebo, n = 209 |
|
| Outcomes | Adverse events | |
| Notes | Oxford Quality Score: R1, DB1, W0. Total = 2. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | Low risk | Treatment groups > 200 participants |
S2BL99.
| Methods | Multicentre, randomised, double‐blind, double‐dummy, parallel‐group. Single dose to treat each of up to 3 attacks. Assessments at 30, 60, and 120 minutes after dosing Second dose of study medication available to treat headache recurrence between 2 and 24 h (second dose could not be taken if the first dose was not effective Rescue medication available after 2 h if response to initial treatment was inadequate |
|
| Participants | Aged 18 to 65, at least 1‐year history of migraine (diagnostic criteria equivalent to IHS 1988) with or without aura, and a frequency of 1 to 6 attacks (untreated severity moderate or severe) per month in the past 12 months No treatment with monoamine oxidase inhibitors or serotonin reuptake inhibitors during the course of the study N = 255 M 52, F 203 (80%) Mean age 43 years Proportion with/without aura not reported |
|
| Interventions | Sumatriptan 6 mg, n = 125 (122 with moderate or severe baseline pain intensity for attack 1) Oral effervescent acetylsalicylic acid (ASA) 1000 mg + metoclopramide (MCP) 10 mg, n = 130 (125 with moderate or severe baseline pain intensity for attack 1) |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 1 and 2 h) Improvement in nausea and vomiting (at 1 and 2 h) Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
S2BM03.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, cross‐over study Each participant received 2 doses; 1 of either sumatriptan or placebo at the onset of migraine and the other at 4 h Assessments at 1, 2, 4, 5, 6, and 72 h after dosing Five optional open‐label doses of sumatriptan 6 mg were available from 6 to 72 h for the treatment of recurrent headache, although no more than 2 doses of sumatriptan were permitted in any 24 h period Rescue medication was permitted from 6 h after the first dose of study medication. No further open‐label sumatriptan was permitted if rescue medication was used. |
|
| Participants | Aged 18 to 65, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate) with a frequency of 1 to 6 attacks per month. Participants required to have a history of attacks (≥ 50% of attacks) that progressed from mild to moderate or severe intensity in ≤ 60 minutes from attack onset In addition participants had to have used sumatriptan regularly for at least 6 months before study entry and experience recurrence in ≥ 50% of attacks treated with sumatriptan At least a 48 h washout period (sumatriptan‐free) required between the 2 treated attacks No ergotamine‐containing prophylactic medication, or use of monoamine oxidase inhibitors, 5‐hydroxtryptamine reuptake inhibitors, or lithium during the study period N = 120 (90 treated both attacks and provided cross‐over efficacy data) M 13, F 77 (86%) Mean age 45 years Proportion with/without aura not reported |
|
| Interventions | Sumatriptan 6 mg (+ placebo at 4 h), n = 106 (90 for cross‐over efficacy analysis, of which 87 had moderate or severe baseline pain intensity) Placebo (+ sumatriptan 6 mg at 4 h), n = 106 (90 for cross‐over efficacy analysis, of which 81 had moderate or severe baseline pain intensity) |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 1 and 2 h) Presence of nausea, vomiting, and photo/phonophobia (at 1 and 2 h) Improvement in functional disability (at 1 and 2 h) Serious adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
S2BS78.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat up to 3 successive attacks. Medication administered at the first sign of headache pain Assessments at 1, 2, 3, 4, 6, 8, and 24 hours after dosing Second injection available to participants after 2 h to treat recurrence of headache or if the response to the initial injection had been inadequate Rescue medication (non‐ergotamine) was permitted 2 h after the second injection |
|
| Participants | Aged 18 to 65, at least 6‐month history of migraine (diagnostic criteria equivalent to IHS 1988) without aura Frequency of 1 to 6 attacks per month in the past 12 months, characterised by slow developing headache (the time interval between onset of mild headache and development of moderate or severe headache had to be consistently greater than 1 hour) N = 349 M 62, F 287 (82%) Mean age 40 years 100% without aura |
|
| Interventions | Sumatriptan 6 mg, n = 136 Placebo, n = 113 |
|
| Outcomes | Serious adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
Sang 2004.
| Methods | Multicentre, randomised, triple‐blind, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 15, 30, 45, 60, and 90 mins and 2, 3, 4, and 24 h after dosing Rescue medication (excluding ergot derivatives) was available at the participant's request after 2 h |
|
| Participants | Aged 18 years or older, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate) with an average of 1 to 15 attacks per month. N = 44 M 20, F 24 (55%) Mean age 40 years Without aura 89% |
|
| Interventions | Sumatriptan 6 mg, n = 15 Intravenous LY293558 1.2 mg/kg, n = 13 Placebo, n = 16 (15 with moderate or severe baseline pain intensity) |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 1 and 2 h) Use of rescue medication Adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Allocation balanced between treatments with a block size equal to 3; randomisation code kept under lock and only accessed by pharmacist or designee |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy |
| Study size | High risk | Treatment groups < 50 participants |
Schulman 2000.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered to treat the next moderate or severe migraine that occurred in the workplace during the first 4 h of an 8 h workday Assessments at 10, 20, 30, 40, 50, 60, 90, and 120 minutes after dosing Rescue medication (excluding ergotamine, ergot‐containing medications or other sumatriptan preparations) available after 2 h if needed |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate) with an average of 1 to 6 attacks per month, and at least 1 debilitating migraine treated in the workplace within 2 months of study enrolment. Participants were required to be employed outside their homes, work a minimum of an 8 h shift, and be willing to self treat a migraine at work with an injection Participants were excluded if they were currently receiving monoamine oxidase inhibitors or had previously taken sumatriptan. Participants were not to have taken any analgesics, antiemetics, or other acute migraine medications within 6 h before use of study medication 140 treated a preliminary attack in clinic N = 119 treated attack in workplace (116 for efficacy) M 14, F 105 (88%) Mean age 40 years Without aura 73% |
|
| Interventions | Sumatriptan 6 mg, n = 76 (for efficacy) Placebo, n = 40 (for efficacy) |
|
| Outcomes | Headache relief (at 1 h) Use of rescue medication Adverse events AE withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W0. Total = 3. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Patients assigned a treatment number in chronological order as they were screened, each treatment number corresponded to a number on the label of unassigned trial medication |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Matching placebo; identical packaging and double‐blind medication labels |
| Study size | High risk | Treatment group 50 to 200 participants, placebo group < 50 participants |
SUM40286.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered within 1 h of awakening with moderate or severe migraine pain, provided the pain continued to be moderate or severe by the time of dosing Assessments at 10, 20, 30, 60, and 120 minutes after dosing Second dose of study medication, up to 100 mg of oral sumatriptan, or alternative rescue medication (usual migraine therapy) was available after 2 h if relief from initial dose was inadequate |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine with 1 to 6 attacks per month, and awakening with at least 1 moderate or severe migraine during the 3 months preceding screening. Participants were excluded if they experienced tension‐type headache on 15 or more days per month in any of the 3 months before screening. Participants had to have successfully treated a migraine attack in the past with a 5‐HT1 agonist, although participants must not have used a subcutaneous formulation of a 5‐HT1 agonist previously N = 299 (297 for efficacy) M 50, F 247 (83%) Mean age 41 years Proportion with/without aura not reported |
|
| Interventions | Sumatriptan 6 mg, n = 146 (145 for efficacy) Placebo, n = 153 (152 for efficacy) |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 1 and 2 h) 24‐hour sustained pain‐free Presence of nausea, vomiting, photophobia, and phonophobia (at 1 and 2 h) Presence of functional disability (at 1 and 2 h) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
SUM40287.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered within 1 h of awakening with moderate or severe migraine pain, provided the pain continued to be moderate or severe by the time of dosing. Assessments at 10, 20, 30, 60, and 120 minutes after dosing. Second dose of study medication, up to 100 mg of oral sumatriptan, or alternative rescue medication (usual migraine therapy) was available after 2 h if relief from initial dose was inadequate. |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine with 1 to 6 attacks per month, and awakening with at least 1 moderate or severe migraine during the 3 months preceding screening. Participants were excluded if they experienced tension‐type headache on 15 or more days per month in any of the 3 months before screening. Participants had to have successfully treated a migraine attack in the past with a 5‐HT1 agonist, although participants must not have used a subcutaneous formulation of a 5‐HT1 agonist previously N = 288 (287 for efficacy) M 38, F 249 (87%) Mean age 39 years Proportion with/without aura not reported |
|
| Interventions | Sumatriptan 6 mg, n = 149 (148 for efficacy) Placebo, n = 139 |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 1 and 2 h) 24‐hour sustained pain‐free Presence of nausea, vomiting, photophobia, and phonophobia (at 1 and 2 h) Presence of functional disability (at 1 and 2 h) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
Thomson 1993.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 30, 60, 90, and 120 minutes after dosing Rescue medication was available after 30 minutes if there was no response to the study treatment |
|
| Participants | To be eligible for entry, participants were required to have a history of migraine (1 to 6 headaches a month) with or without aura as defined by the IHS (1988) No narcotic analgesics or ergotamine within the previous 24 h, or aspirin within the previous 6 h before study treatment N = 51 (50 for efficacy) M 7, F 43 (86%) Mean age 41 years Without aura 74% |
|
| Interventions | Sumatriptan 4 mg, n = 28 Placebo, n = 23 (22 for efficacy) |
|
| Outcomes | Only 30 minute efficacy outcomes reported Adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Matching placebo |
| Study size | High risk | Treatment groups < 50 participants |
Touchon 1996.
| Methods | Multicentre, randomised, double‐blind, double‐dummy, cross‐over design. Single dose to treat each of 2 successive attacks. Assessments at 15, 30, 60, 90, and 120 minutes after dosing Participants randomised to the dihydroergotamine (DHE) treatment arm had the option of a second dose of study medication after 30 minutes if their relief was inadequate. Participants in the sumatriptan treatment arm were offered a second dose of placebo after 30 minutes. Rescue medication (excluding ergotamine‐containing medications, DHE, or sumatriptan) available after 2 h if migraine symptoms not adequately relieved |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate) with 1 to 6 attacks per month. Prophylactic treatment for migraine, with the exception of oral DHE, was allowed provided dosage remained unchanged during the study N = 289 (266 treated both attacks) M 36, F 230 (86%) Mean age 42 years Proportion with/without aura not reported Baseline pain intensity not reported; participants normally experiencing moderate or severe attacks were recruited but it is likely that some of the treated participants will have had mild baseline pain intensity |
|
| Interventions | Sumatriptan 6 mg, n = 278 (145 treated first attack, 266 in cross‐over analysis) DHE nasal spray 1 mg, n = 277 (144 treated first attack, 266 in cross‐over analysis) |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 1 and 2 h) 24 h sustained headache relief Improvement in nausea at 2 h Improvement in functional disability at 2 h Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy |
| Study size | Low risk | Treatment groups > 200 participants |
Visser 1992.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 30, 60, and 120 minutes after dosing An open 3 mg injection of sumatriptan was available after 30 minutes if headache had not improved to no worse than mild Rescue medication (not containing ergotamine or dihydroergotamine) was available after 60 minutes if relief remained inadequate |
|
| Participants | Aged 18 to 60 years, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate) with 1 to 6 attacks per month. No use of ergotamine or morphine‐containing preparations within 24 h, or analgesics within 6 h of study treatment The use of prophylactic therapy, provided it did not contain ergotamine, was allowed N = 685 (672 for efficacy) M 165, F 520 (76%) Mean age 40 years Without aura 76% |
|
| Interventions | Sumatriptan 1 mg, n = 170 Sumatriptan 2 mg, n = 171 Sumatriptan 3 mg, n = 172 Placebo, n = 172 |
|
| Outcomes | Efficacy data only reported for 30 minutes Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
Wendt 2006.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 10, 20, 30, 40, 50, 60, 90, and 120 minutes after dosing Rescue medication available after 1 h if needed, although participants using rescue medication were counted as treatment failures from the time it was given |
|
| Participants | Aged 18 to 60 years, meeting IHS criteria for migraine (1988) with or without aura Participants were excluded if they had previous exposure to sumatriptan No use of analgesics containing morphine or ergotamine within the preceding 24 h, simple analgesics within the preceding 6 h, or any acute illness requiring the administration of a prescription drug within 24 h of starting the study Normal migraine prophylaxis was allowed N = 577 (572 with moderate or severe baseline pain intensity) M 76, F 501 (87%) Mean age 38 years Without aura 66% |
|
| Interventions | Sumatriptan 4 mg, n = 384 (381 with moderate or severe baseline pain intensity) Placebo, n = 193 (191 with moderate or severe baseline pain intensity) |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 1 and 2 h) Improvement in nausea and photophobia at 2 h Use of rescue medication Adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W0. Total = 3. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study medications were provided in indistinguishable clear glass ampoules labelled with an overleaf that served to blind investigators and participants |
| Study size | Unclear risk | Treatment group > 200 participants, placebo group 50 to 200 participants |
Winner 1996.
| Methods | Multicentre, randomised, double‐blind, parallel‐group. Single dose to treat single attack. Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 0.5, 1, 2, 2,5, 3, 4, and 24 h after dosing Second dose of study medication available after 2 h for those who had not obtained relief Rescue medication (excluding ergotamine, dihydroergotamine, sumatriptan, or steroids) available 1 h after second injection if relief was still inadequate At the 1 h evaluation, intramuscular prochlorperazine edisylate (10 mg) or metoclopramide hydrochloride (10 mg) could be given for vomiting |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate) with 1 to 6 attacks per month. Prophylactic medication for migraine was permitted providing there were no changes in the medication for at least 2 weeks before study dosing Participants were excluded if experienced aura phase with a duration longer than 1 h, were currently using serotonin reuptake inhibitors, or if they used opioid or other analgesics for more than 3 days per week The use of any form of ergot alkaloid or sumatriptan was prohibited in the 72 h preceding study drug administration, as well as use of antiemetics and narcotic analgesics in the 24 h preceding administration N = 310 M 38, F 272 (88%) Mean age 41 years Migraine without aura was the principal headache diagnosis Although all participants had moderate or severe baseline pain intensity, there was a difference in the distribution of moderate and severe pain between groups, therefore the authors adjusted pain ratings for baseline values (no further details) |
|
| Interventions | Sumatriptan 6 mg, n = 158 (150 for efficacy) Subcutaneous dihydroergotamine (DHE) mesylate 1 mg, n = 152 (145 for efficacy) |
|
| Outcomes | Headache relief (at 1 and 2 h) Improvement in nausea and vomiting at 1 h Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
Winner 2006.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered to treat a morning migraine (defined as a headache of moderate or severe intensity on awakening) within 1 hour of awakening Assessments at 10, 20, 30, 60, and 120 minutes after dosing Second dose of study medication or alternative rescue medication available after 2 h for participants with inadequate relief or for those experiencing recurrence within 24 h 2 identically designed studies: Study 1 and Study 2 |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine with 1 to 6 attacks per month, and had awakened with moderate or severe migraine pain at least once in the 3 months preceding screening. No migraine prophylactic medication containing ergotamine, an ergot derivative, or methysergide, and no use of a monoamine oxidase inhibitor within 2 weeks before the studies. Participants were eligible for the studies only if they had previously treated a migraine successfully with a 5‐HT1B/1D agonist, but participants who had previously used subcutaneous sumatriptan were excluded No analgesics, antiemetics, or acute migraine medications from 6 h before through to 2 h after administration of study medication. No other 5‐HT agonists within 24 h before or after use of study medication, and no ergotamine or ergot‐type medications (including methysergide) for the duration of the studies Study 1 N = 299 (297 for efficacy) M 50, F 247 (83%) Mean age 41 years Without aura 61% Study 2 N = 288 (287 for efficacy) M 38, F 249 (87%) Mean age 39 years Without aura 73% |
|
| Interventions | Study 1 Sumatriptan 6 mg, n = 146 (145 for efficacy, 144 with moderate or severe baseline pain intensity) Placebo, n = 153 (152 for efficacy, 151 with moderate or severe baseline pain intensity) Study 2 Sumatriptan 6 mg, n = 149 (148 for efficacy) Placebo, n = 139 |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 2 h) 24 h sustained pain‐free Improvement in nausea, vomiting, photophobia, and phonophobia at 2 h Improvement in functional disability at 2 h Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Remote allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Matching inactive vehicle injection in identical prefilled single‐dose syringe cartridges |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
All medication delivered subcutaneously unless otherwise stated
AE: adverse event; DB: double‐blinding; DHE: dihydroergotamine; GP: general practitioner; h: hour; IHS: International Headache Society; NSAIDs: non‐steroidal anti‐inflammatory drugs; R: randomisation; W: withdrawals
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Burke‐Ramirez 2001 | Number of participants in each treatment arm not reported and no indication of baseline pain intensity for any treated participants |
| Cady 1994 | First dose of subcutaneous sumatriptan not randomised, only for subsequent doses of oral sumatriptan for recurrence (from 2 to 24 h after initial dosing) were patients randomised to either sumatriptan or placebo |
| Cull 2001 | All participants initially treat with sumatriptan at the onset of migraine headache, and are only randomised to either sumatriptan or placebo to treat any subsequent recurrence that occurred between 1 and 24 h after the first dose was administered |
| Ensink 1991 | 2 studies: Study 1 ‐ Baseline pain intensity of treated participants not reported and at least 50% of participants in each treatment arm took a second dose of study medication at 30 minutes. No useable efficacy data at 1 or 2 h and no adverse event data reported. Study 2 ‐ Data reported in Mathew 1992 |
| Friedman 2005 | Only comparator (intravenous metoclopramide 20 mg) was not self administrable. No placebo group. |
| Friedman 2006 | Only comparator (intramuscular combination of trimethobenzamide 200 mg + diphenhydramine 25 mg) was not self administrable. No placebo group. |
| Gonzalez‐Espinosa 1997 | Only comparator (intramuscular dihydroergotamine 1 mg) was not self administrable. No placebo group. In addition, blinding of study medication is uncertain (study does not appear to use double‐dummy technique) and the baseline pain intensity of treated participants is not reported |
| Melchart 2003 | Non‐standard pain scale (50‐point categorical scale) and use of an additional dose of sumatriptan by the majority of participants at unknown, variable time point (any time after initial dosing if participants developed a full migraine attack: ˜60% used 2nd dose) meaning no useable efficacy or safety data |
| Pradel 2006 | Not subcutaneous route of administration |
| Russell 1995 | Data reported in Russell 1994 |
| S2BM04 | All participants initially treated with oral sumatriptan 100 mg; only those failing to respond to this initial treatment were subsequently randomised to receive either subcutaneous sumatriptan 4 mg or placebo |
| Solbach 1993 | Subgroup analysis of data reported in Cady 1991 for menstruation‐associated migraine. No additional data reported. |
H: hour
Differences between protocol and review
We have considered data for two outcomes not specified in the protocol.
Use of rescue medication was reported by the majority of studies, and provides a measure of efficacy from the point of view of the patient. In taking rescue medication the patient is saying that the efficacy of the medication is not adequate and that they need alternative analgesia. They are effectively withdrawing due to lack of efficacy, where efficacy is defined by their preparedness to carry on without additional analgesia, rather than a predefined outcome such as headache relief at two hours. We believe this is useful additional information relevant to clinical practice.
Pain‐free at one hour provides, along with headache relief at one hour, a measure of the speed of onset of the medication. This is an important feature of some anti‐migraine treatments and can vary significantly between different routes of administration of the same drug. We chose to analyse pain‐free at one hour to provide a stringent measure of the early efficacy of subcutaneous sumatriptan, which we believe to be important information for clinical practice.
We have included data for withdrawals due to adverse events over reporting periods longer than the 24 hours stated in the protocol. Many studies collected adverse event data for longer than 24 hours after treatment, and it is likely that in these cases data on withdrawals due to adverse events were also collected over longer time periods. Adverse event withdrawals were infrequent in all of the trials reporting, regardless of the time period over which they were collected, but are an important measure of drug safety and tolerability. We therefore decided to be as inclusive as possible with data on adverse event withdrawals, in the hope of providing the most comprehensive picture possible of sumatriptan tolerability.
For calculations of susceptibility to publication bias we have used a NNT of ≥ 8 as the limit of clinical utility for pain‐free at two hours and ≥ 6 for headache relief at two hours. In the protocol we said we would use a NNT of ≥ 8 for headache relief at two hours, but made the change following a discussion with the field editor.
Contributions of authors
SD and RAM wrote the protocol. CD and SD carried out searches, data extraction, and analyses. RAM acted as arbitrator. All authors were involved with writing the final review.
Sources of support
Internal sources
Oxford Pain Research funds, UK.
External sources
Cochrane Review Incentive Scheme 2010, UK.
-
Lifting The Burden: the Global Campaign against Headache, UK.
Funding for administrative costs associated with editorial and peer review
Declarations of interest
RAM and SD have received research support from charities, government, and industry sources at various times. RAM has consulted for various pharmaceutical companies, including GlaxoSmithKline, the manufacturers of sumatriptan. RAM has received lecture fees from pharmaceutical companies related to analgesics and other healthcare interventions. CD has no interests to declare. GlaxoSmithKline were not in any way involved in carrying out this review.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Akpunonu 1995 {published data only}
- Akpunonu BE, Mutgi AB, Federman DJ, Volinsky FG, Brickman K, Davis RL, et al. Subcutaneous sumatriptan for treatment of acute migraine in patients admitted to the emergency department: a multicenter study. Annals of Emergency Medicine 1995;25(4):464‐9. [DOI] [PubMed] [Google Scholar]
Bates 1994 {published data only}
- Bates D, Ashford E, Dawson R, Ensink FB, Gilhus NE, Olesen J, et al. Sumatriptan Aura Study Group. Subcutaneous sumatriptan during the migraine aura. Neurology 1994;44(9):1587‐92. [DOI] [PubMed] [Google Scholar]
Bousser 1993 {published data only}
- Bousser MG, D'Allens H, Richard A. Efficacy of subcutaneous sumatriptan in the acute treatment of early‐morning migraine: a placebo‐controlled trial. Journal of Internal Medicine 1993;234(2):211‐6. [DOI] [PubMed] [Google Scholar]
Cady 1991 {published data only}
- Cady RK, Wendt JK, Kirchner JR, Sargent JD, Rothrock JF, Skaggs H Jr. Treatment of acute migraine with subcutaneous sumatriptan. JAMA 1991;265(21):2831‐5. [PubMed] [Google Scholar]
Cady 1993 {published data only}
- Repeat‐dose efficacy and laboratory safety trial of subcutaneous GR43175C in migraine patients. http://www.gsk‐clinicalstudyregister.com/.
- Cady RK, Dexter J, Sargent JD, Markley H, Osterhaus JT, Webster CJ. Efficacy of subcutaneous sumatriptan in repeated episodes of migraine. Neurology 1993;43(7):1363‐8. [DOI] [PubMed] [Google Scholar]
Cady 1998 {published data only}
- Cady RC, Ryan R, Jhingran P, O'Quinn S, Pait DG. Sumatriptan injection reduces productivity loss during a migraine attack: results of a double‐blind, placebo‐controlled trial. Archives of Internal Medicine 1998;158(9):1013‐8. [DOI] [PubMed] [Google Scholar]
Dahlof 1992 {published data only}
- Dahlof C, Edwards C, Toth A. Sumatriptan injection is superior to placebo in the acute treatment of migraine‐‐with regard to both efficacy and general well‐being. Cephalalgia 1992;12(4):214‐20. [DOI] [PubMed] [Google Scholar]
Dahlof 1998 {published data only}
- Dahlof C, Hogenhuis L, Olesen J, Petit H, Ribbat J, Schoenen J, et al. Early clinical experience with subcutaneous naratriptan in the acute treatment of migraine: a dose‐ranging study. European Journal of Neurology 1998;5(5):469‐77. [DOI: 10.1046/j.1468-1331.1998.550469.x] [DOI] [PubMed] [Google Scholar]
Diener 1999 {published data only}
- Diener HC. Efficacy and safety of intravenous acetylsalicylic acid lysinate compared to subcutaneous sumatriptan and parenteral placebo in the acute treatment of migraine. A double‐blind, double‐dummy, randomized, multicenter, parallel group study. Cephalalgia 1999;19(6):581‐8. [DOI] [PubMed] [Google Scholar]
Diener 2001 {published data only}
- Diener HC, Tfelt‐Hansen P, Beukelaar F, Ferrari MD, Olesen J, Dahlof C, et al. The efficacy and safety of sc alniditan vs. sc sumatriptan in the acute treatment of migraine: a randomized, double‐blind, placebo‐controlled trial. Cephalalgia 2001;21(6):672‐9. [DOI: ] [DOI] [PubMed] [Google Scholar]
Facchinetti 1995 {published data only}
- Facchinetti F, Bonellie G, Kangasniemi P, Pascual J, Shuaib A, The Sumatriptan Menstrual Migraine Study Group. The efficacy and safety of subcutaneous sumatriptan in the acute treatment of menstrual migraine. Journal of Obstetrics and Gynecology 1995;86(6):911‐6. [PUBMED: 7501338] [DOI] [PubMed] [Google Scholar]
Ferrari 1991 {published data only}
- The Subcutaneous Sumatriptan International Study Group. Treatment of migraine attacks with sumatriptan. New England Journal of Medicine 1991;325(5):316‐21. [DOI] [PubMed] [Google Scholar]
Gross 1994 {published data only}
- Gross ML, Kay J, Turner AM, Hallett K, Cleal AL, Hassani H, United Kingdom Study Group. Sumatriptan in acute migraine using a novel cartridge system self‐injector. Headache 1994;34(10):559‐63. [PUBMED: 9843948] [DOI] [PubMed] [Google Scholar]
Henry 1993 {published data only}
- Henry P, d'Allens H, French Migraine Network Bordeaux‐Lyon‐Grenoble. Subcutaneous sumatriptan in the acute treatment of migraine in patients using dihydroergotamine as prophylaxis. Headache 1993;33(8):432‐5. [DOI] [PubMed] [Google Scholar]
Jensen 1995 {published data only}
- Jensen K, Tfelt‐Hansen P, Hansen EW, Krøis EH, Pedersen OS. Introduction of a novel self‐injector for sumatriptan. A controlled clinical trial in general practice. Cephalalgia 1995;15(5):423‐9. [DOI] [PubMed] [Google Scholar]
Mathew 1992 {published data only}
- Mathew NT, Dexter J, Couch J, Flamenbaum W, Goldstein J, Rapoport A, et al. US Sumatriptan Research Group. Dose ranging efficacy and safety of subcutaneous sumatriptan in the acute treatment of migraine. Archives of Neurology 1992;49(12):1271‐6. [DOI] [PubMed] [Google Scholar]
Mushet 1996 {published data only}
- Mushet GR, Cady RK, Baker CC, Clements B, Gutterman DL, Davis R. Efficacy and tolerability of subcutaneous sumatriptan administered using the IMITREX STATdose System. Clinical Therapeutics 1996;18(4):687‐99. [DOI] [PubMed] [Google Scholar]
Pfaffenrath 1991 {published data only}
- The Sumatriptan Auto‐Injector Study Group. Self‐treatment of acute migraine with subcutaneous sumatriptan using an auto‐injector device. European Neurology 1991;31(5):323‐31. [DOI] [PubMed] [Google Scholar]
Russell 1994 {published data only}
- Russell MB, Holm‐Thomsen OE, Rishøj Nielsen M, Cleal A, Pilgrim AJ, Olesen J. A randomized double‐blind placebo‐controlled crossover study of subcutaneous sumatriptan in general practice. Cephalalgia 1994;14(4):291‐6. [DOI] [PubMed] [Google Scholar]
S2BL99 {unpublished data only}
- A randomised, multicentre, double‐blind, double‐dummy, parallel‐group study to compare the efficacy and safety of subcutaneous sumatriptan with oral aspirin plus oral metoclopramide in the acute treatment of migraine. http://www.gsk‐clinicalstudyregister.com/.
S2BM03 {unpublished data only}
- A double‐blind, randomised, placebo controlled, crossover study to assess return of headache in patients treated with 6 mg subcutaneous sumatriptan early or late in a migraine attack; with optional open‐label doses to assess pattern of use of sumatriptan over the subsequent 72 hour period. http://www.gsk‐clinicalstudyregister.com/.
S2BS78 {unpublished data only}
- A double‐blind, placebo‐controlled, parallel group study of 6 mg subcutaneous sumatriptan + optional 6 mg subcutaneous sumatriptan administered using a novel cartridge system self‐injector in slow developing migraine. http://www.gsk‐clinicalstudyregister.com/.
Sang 2004 {published data only}
- Sang CN, Ramadan NM, Wallihan RG, Chappell AS, Freitag FG, Smith TR, et al. LY293558, a novel AMPA/GluR5 antagonist, is efficacious and well‐tolerated in acute migraine. Cephalalgia 2004;24(7):596‐602. [DOI] [PubMed] [Google Scholar]
Schulman 2000 {published data only}
- Schulman EA, Cady RK, Henry D, Batenhorst AS, Putnam DG, Watson CB, et al. Effectiveness of sumatriptan in reducing productivity loss due to migraine: results of a randomized, double‐blind, placebo‐controlled clinical trial. Mayo Clinic Proceedings 2000;75(8):782‐9. [DOI] [PubMed] [Google Scholar]
SUM40286 {published and unpublished data}
- A randomized, double‐blind, placebo‐controlled, parallel‐group, single‐attack study of sumatriptan 6 mg injection in the treatment of moderate‐to‐severe migraine present upon awakening. http://www.gsk‐clinicalstudyregister.com/.
- Winner P, Adelman J, Aurora S, Ames M, Lener M. Pain‐free results of sumatriptan 6 mg SC in the treatment or morning migraine: results from two prospective, randomized, double‐blind, placebo‐controlled studies. Headache 2003;43(5):535. [Google Scholar]
SUM40287 {published and unpublished data}
- A randomized, double‐blind, placebo‐controlled, parallel‐group, single‐attack study of sumatriptan 6 mg injection in the treatment of moderate‐to‐severe migraine present upon awakening. http://www.gsk‐clinicalstudyregister.com/.
- Winner P, Adelman J, Aurora S, Ames M, Lener M. Pain‐free results of sumatriptan 6 mg SC in the treatment or morning migraine: results from two prospective, randomized, double‐blind, placebo‐controlled studies. Headache 2003;43(5):535. [Google Scholar]
Thomson 1993 {published data only}
- Thomson AN, Arthur GP, Bergin PS, Pollock M, Parkin PJ, Flanagan M, et al. Subcutaneous sumatriptan in acute treatment of migraine: a multicentre New Zealand trial. New Zealand Medical Journal 1993;106(955):171‐3. [PubMed] [Google Scholar]
Touchon 1996 {published data only}
- Touchon J, Bertin L, Pilgrim AJ, Ashford E, Bès A. A comparison of subcutaneous sumatriptan and dihydroergotamine nasal spray in the acute treatment of migraine. Neurology 1996;47(2):361‐5. [DOI] [PubMed] [Google Scholar]
Visser 1992 {published data only}
- Visser WH, Ferrari MD, Bayliss EM, Ludlow S, Pilgrim AJ, The Subcutaneous Sumatriptan International Study Group. Treatment of migraine attacks with subcutaneous sumatriptan: first placebo‐controlled study. Cephalalgia 1992;12(5):308‐13. [DOI] [PubMed] [Google Scholar]
Wendt 2006 {published data only}
- Wendt J, Cady R, Singer R, Peters K, Webster C, Kori S, et al. A randomized, double‐blind, placebo‐controlled trial of the efficacy and tolerability of a 4‐mg dose of subcutaneous sumatriptan for the treatment of acute migraine attacks in adults. Clinical Therapeutics 2006;28(4):517‐26. [DOI: 10.1016/j.clinthera.2006.03.013] [DOI] [PubMed] [Google Scholar]
Winner 1996 {published data only}
- Winner P, Mannix LK, Putnam DG, McNeal S, Kwong J, O'Quinn S, et al. Pain‐free results with sumatriptan taken at the first sign of migraine pain: 2 randomized, double‐blind, placebo‐controlled studies. Mayo Clinic Proceedings 2003 ;78(10):1214‐22. [DOI: 10.4065/78.10.1214] [DOI] [PubMed] [Google Scholar]
Winner 2006 {published data only}
- Winner P, Adelman J, Aurora S, Lener ME, Ames M. Efficacy and tolerability of sumatriptan injection for the treatment of morning migraine: two multicenter, prospective, randomized, double‐blind, controlled studies in adults. Clinical Therapeutics 2006;28(10):1582‐91. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Burke‐Ramirez 2001 {published data only}
- Burke‐Ramirez P, Asgharnejad M, Webster C, Davis R, Laurenza A. Efficacy and tolerability of subcutaneous sumatriptan for acute migraine: a comparison between ethnic groups. Headache 2001;41(9):873‐82. [DOI: 10.1111/j.1526-4610.2001.01159.x] [DOI] [PubMed] [Google Scholar]
Cady 1994 {published data only}
- Cady RK, Rubino J, Crummett D, Littlejohn TW 3rd. Oral sumatriptan in the treatment of recurrent headache. Archives of Family Medicine 1994;3(9):766‐72. [DOI] [PubMed] [Google Scholar]
Cull 2001 {published data only}
- Cull RE, Price WH, Dunbar A. The efficacy of subcutaneous sumatriptan in the treatment of recurrence of migraine headache. Journal of Neurology, Neurosurgery, and Psychiatry 1997;62(5):490‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ensink 1991 {published data only}
- Ensink FB. Subcutaneous sumatriptan in the acute treatment of migraine. Sumatriptan International Study Group. Journal of Neurology 1991;238 Suppl 1:S66‐9. [DOI] [PubMed] [Google Scholar]
Friedman 2005 {published data only}
- Friedman BW, Corbo J, Lipton RB, Bijur PE, Esses D, Solorzano C, et al. A trial of metoclopramide vs sumatriptan for the emergency department treatment of migraines. Neurology 2005;64(3):463‐8. [DOI] [PubMed] [Google Scholar]
Friedman 2006 {published data only}
- Friedman BW, Hochberg M, Esses D, Bijur PE, Corbo J, Paternoster J, et al. A clinical trial of trimethobenzamide/diphenhydramine versus sumatriptan for acute migraines. Headache 2006;46(6):934‐41. [DOI: 10.1111/j.1526-4610.2006.00467.x] [DOI] [PubMed] [Google Scholar]
Gonzalez‐Espinosa 1997 {published data only}
- González‐Espinosa LE, Gómez‐Viera N, Olivera‐Leal I, Reyes‐Lorente R. Treatment of acute attack of migraine with sumatriptan [Spanish]. Revista de Neurologia 1997;147:1672‐5. [PubMed] [Google Scholar]
Melchart 2003 {published data only}
- Melchart D, Thormaehlen J, Hager S, Liao J, Linde K, Weidenhammer W. Acupuncture versus placebo versus sumatriptan for early treatment of migraine attacks: a randomized controlled trial. Journal of Internal Medicine 2003;253(2):181‐8. [DOI: 10.1046/j.1365-2796.2003.01081.x] [DOI] [PubMed] [Google Scholar]
Pradel 2006 {published data only}
- Pradel FG, Subedi P, Varghese AA, Mullins CD, Weis KA. Does earlier headache response equate to earlier return to functioning in patients suffering from migraine?. Cephalalgia 2006;26(4):428‐35. [DOI] [PubMed] [Google Scholar]
Russell 1995 {published data only}
- Russell MB, Holm‐Thomsen OE, Nielsen MR, Cleal A, Pilgrim AJ, Olesen J. Sumatriptan treatment of migraine in general practice. A randomized, double‐blind, placebo‐controlled cross‐over study [Sumatriptan ved migraene i almen praksis. En randomiseret, dobbeltblind, placebokontrolleret overkrydsningsundersogelse]. Ugeskrift for Laeger 1995;157(16):2320‐3. [PubMed] [Google Scholar]
S2BM04 {unpublished data only}
- A double‐blind, placebo‐controlled, parallel group study to evaluate the efficacy, safety and tolerability of a 6 mg SC dose of sumatriptan in patients who have not responded to acute treatment of a migraine attack with 100 mg oral dose of sumatriptan. http://www.gsk‐clinicalstudyregister.com/.
Solbach 1993 {published data only}
- Solbach MP, Waymer RS. Treatment of menstruation‐associated migraine headache with subcutaneous sumatriptan. Journal of Obstetrics and Gynecology 1993;82(5):769‐72. [PubMed] [Google Scholar]
Additional references
Bigal 2008
- Bigal ME, Serrano D, Reed M, Lipton RB. Chronic migraine in the population: burden, diagnosis, and satisfaction with treatment. Neurology 2008;71(8):559‐66. [DOI: 10.1212/01.wn1.0000323925.29520.e7] [DOI] [PubMed] [Google Scholar]
Clarke 1996
- Clarke CE, MacMillan L, Sondhi S, Wells NEJ. Economic and social impact of migraine. Quarterly Journal of Medicine 1996;89(1):77‐84. [DOI] [PubMed] [Google Scholar]
Cook 1995
- Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ 1995;310(6977):452‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry (forthcoming)
- Derry CJ, Derry S, Moore RA. Sumatriptan (all routes of administration) for acute migraine attacks in adults: an overview of Cochrane reviews. Cochrane Database of Systematic Reviews (forthcoming). [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2012a
- Derry CJ, Derry S, Moore RA. Sumatriptan (oral route of administration) for acute migraine attacks in adults. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD008615] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2012b
- Derry CJ, Derry S, Moore RA. Sumatriptan (subcutaneous route of administration) for acute migraine attacks in adults. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD009665] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2012c
- Derry CJ, Derry S, Moore RA. Sumatriptan (intranasal route of administration) for acute migraine attacks in adults. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD009663] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2012d
- Derry CJ, Derry S, Moore RA. Sumatriptan (rectal route of administration) for acute migraine attacks in adults. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD009664] [DOI] [PMC free article] [PubMed] [Google Scholar]
Diamond 2007
- Diamond S, Bigal ME, Silberstein S, Loder E, Reed M, Lipton RB. Patterns of diagnosis and acute and preventive treatment for migraine in the United States: results from the American Migraine Prevalence and Prevention study. Headache 2007;47(3):355‐63. [DOI: 10.1111/j.1526-4610.2006.00631.x] [DOI] [PubMed] [Google Scholar]
Edmeads 1993
- Edmeads J, Findlay H, Tugwell P, Pryse‐Phillips W, Nelson RF, Murray TJ. Impact of migraine and tension‐type headache on life‐style, consulting behaviour, and medication use: a Canadian population survey. Canadian Journal of Neurological Sciences 1993;20(2):131‐7. [DOI] [PubMed] [Google Scholar]
Ferrari 1998
- Ferrari MD. The economic burden of migraine to society. Pharmacoeconomics 1998;13(6):667‐76. [DOI] [PubMed] [Google Scholar]
Ferrari 2002
- Ferrari MD, Goadsby PJ, Roon KI, Lipton RB. Triptans (serotonin, 5‐HT1B/1D agonists) in migraine: detailed results and methods of a meta‐analysis of 53 trials. Cephalalgia 2002;22(8):633‐58. [DOI: 10.1046/j.1468-2982.2002.00404.x] [DOI] [PubMed] [Google Scholar]
Gawel 2001
- Gawel MJ, Worthington I, Maggisano A. A systematic review of the use of triptans in acute migraine. Canadian Journal of Neurological Sciences 2001;28(1):30‐41. [DOI] [PubMed] [Google Scholar]
Gendolla 2008
- Gendolla A. Early treatment in migraine: how strong is the current evidence?. Cephalalgia 2008;28 Suppl 2:28‐35. [DOI] [PubMed] [Google Scholar]
Goadsby 2007
- Goadsby PJ. Recent advances in understanding migraine mechanisms, molecules and therapeutics. Trends in Molecular Medicine 2007;13(1):39‐44. [DOI: 10.1016/j.molmed.2006.11.005] [DOI] [PubMed] [Google Scholar]
Hazard 2009
- Hazard E, Munakata J, Bigal ME, Rupnow MF, Lipton RB. The burden of migraine in the United States: current and emerging perspectives on disease management and economic analysis. Value in Health 2009;12(1):55‐64. [DOI: 10.1111/j.1524-4733.2008.00404.x] [DOI] [PubMed] [Google Scholar]
Hu 1999
- Hu XH, Markson LE, Lipton RB, Stewart WF, Berger ML. Burden of migraine in the United States: disability and economic costs. Archives of Internal Medicine 1999;159(8):813‐8. [DOI] [PubMed] [Google Scholar]
IHS 1988
- Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia 1988;8 Suppl 7:1‐96. [PubMed] [Google Scholar]
IHS 2000
- International Headache Society Clinical Trials Subcommittee. Guidelines for controlled trials of drugs in migraine: second edition. Cephalalgia 2000;20(9):765‐86. [DOI] [PubMed] [Google Scholar]
IHS 2004
- Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004;24 Suppl 1:1‐160. [DOI] [PubMed] [Google Scholar]
Jadad 1996a
- Jadad AR, Carroll D, Moore A, McQuay H. Developing a database of published reports of randomised clinical trials in pain research. 1996 1996;66(2‐3):239‐46. [DOI] [PubMed] [Google Scholar]
Jadad 1996b
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
Jhingran 1996
- Jhingran P, Cady RK, Rubino J, Miller D, Grice RB, Gutterman DL. Improvements in health‐related quality of life with sumatriptan treatment for migraine. Journal of Family Practice 1996;42(1):36‐42. [PubMed] [Google Scholar]
L'Abbé 1987
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107(2):224‐33. [DOI] [PubMed] [Google Scholar]
Lipton 1999
- Lipton RB, Stewart WF. Acute migraine therapy: do doctors understand what patients with migraine want from therapy?. Headache 1999;39 Suppl 2:S20‐S26. [Google Scholar]
Lipton 2007
- Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, AMPP Advisory Group, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007;68(5):343‐9. [DOI] [PubMed] [Google Scholar]
Lofland 1999
- Lofland JH, Johnson NE, Batenhorst AS, Nash DB. Changes in resource use and outcomes for patients with migraine treated with sumatriptan: a managed care perspective. Archives of Internal Medicine 1999;159(8):857‐63. [DOI] [PubMed] [Google Scholar]
Lucas 2006
- Lucas C, Géraud G, Valade D, Chautard MH, Lantéri‐Minet M. Recognition and therapeutic management of migraine in 2004, in France: results of FRAMIG 3, a French nationwide population‐based survey. Headache 2006;46(5):715‐25. [DOI: 10.1111/j.1526-4610.2006.00430.x] [DOI] [PubMed] [Google Scholar]
McCrory 2003
- McCrory DC, Gray RN. Oral sumatriptan for acute migraine. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD002915] [DOI] [PubMed] [Google Scholar]
Moore 1998
- Moore RA, Gavaghan D, Tramèr MR, Collins SL, McQuay HJ. Size is everything ‐ large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209‐16. [DOI] [PubMed] [Google Scholar]
Moore 2008
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15‐24. [Google Scholar]
Moore 2010
- Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, et al. ‘‘Evidence” in chronic pain – establishing best practice in the reporting of systematic reviews. Pain 2010;150(3):386–9. [DOI] [PubMed] [Google Scholar]
Morris 1995
- Morris JA, Gardner MJ. Calculating confidence intervals for relative risk, odds ratios and standardised ratios and rates. In: Gardner MJ, Altman DG editor(s). Statistics with Confidence ‐ Confidence Intervals and Statistical Guidelines. London: British Medical Journal, 1995:50‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oldman 2002
- Oldman AD, Smith LA, McQuay HJ, Moore RA. Pharmacological treatments for acute migraine: quantitative systematic review. Pain 2002;97(3):247‐57. [DOI] [PubMed] [Google Scholar]
Osterhaus 1994
- Osterhaus JT, Townsend RJ, Gandek B, Ware JE Jr. Measuring the functional status and well‐being of patients with migraine headache. Headache 1994;34(6):337‐43. [DOI] [PubMed] [Google Scholar]
Pascual 2002
- Pascual J. Clinical benefits of early triptan therapy for migraine. Headache 2002;42 Suppl 1:10‐7. [DOI: 10.1046/j.1526-4610.2002.0420s1010.x] [DOI] [PubMed] [Google Scholar]
PCA 2011
- Anonymous. Prescription cost analysis, England 2010. The NHS Information Centre, Prescribing Support Unit 2011. [ISBN: 978‐1‐84636‐542‐3]
Radtke 2009
- Radtke A, Neuhauser H. Prevalence and burden of headache and migraine in Germany. Headache 2009;49(1):79‐89. [DOI: 10.1111/j.1526-4610.2008.01263.x] [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Solomon 1997
- Solomon GD, Price KL. Burden of migraine. A review of its socioeconomic impact. Pharmacoeconomics 1997;11 Suppl 1:1‐10. [DOI] [PubMed] [Google Scholar]
Stovner 2010
- Stovner LJ, Andree C. Prevalence of headache in Europe: a review for the Eurolight project. Journal of Headache and Pain 2010;11(4):289‐99. [DOI: 10.1007/s10194-010-0217-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tfelt‐Hansen 1998
- Tfelt‐Hansen P. Efficacy and adverse events of subcutaneous, oral, and intranasal sumatriptan used for migraine treatment: a systematic review based on number needed to treat. Cephalalgia 1998;18(8):532‐8. [DOI] [PubMed] [Google Scholar]
Tramer 1997
- Tramèr MR, Reynolds DJM, Moore RA, McQuay HJ. Impact of covert duplicate results on meta‐analysis: a case study. BMJ 1997;315(7109):635‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Victor 2010
- Victor TW, Hu X, Campbell JC, Buse DC, Lipton RB. Migraine prevalence by age and sex in the United States: a life‐span study. Cephalalgia 2010;30(9):1065‐72. [DOI: 10.1177/0333102409355601] [DOI] [PubMed] [Google Scholar]


