In Australia, most Aboriginal and Torres Strait Islander (ATSI) patients reside away from city centers. Therefore, for those patients, peritoneal dialysis (PD) is often a preferred modality of treatment for end-stage kidney disease (ESKD). A recent analysis of the ANZDATA Registry revealed that, of the indigenous patients initiated on PD, a greater proportion lived in remote locations (79%) than in regional (9%) or metropolitan (8%) areas (1).
Far North Queensland is the northernmost part of the Australian state of Queensland. The region stretches from the city of Cairns to the Torres Strait and covers an area of 27 3147 km2 (2). The provision of renal services is administered by Cairns Base Hospital (Figure 1). Before 2003, the process of securing PD access involved travel to that facility, coordination with surgeons and anesthetists, and organization of a hospital bed and an operating theatre session. Those logistical constraints can affect the timely insertion of the PD catheter, a critical part of a successful PD program that can be influenced by interventional nephrologists (3).
Figure 1 —
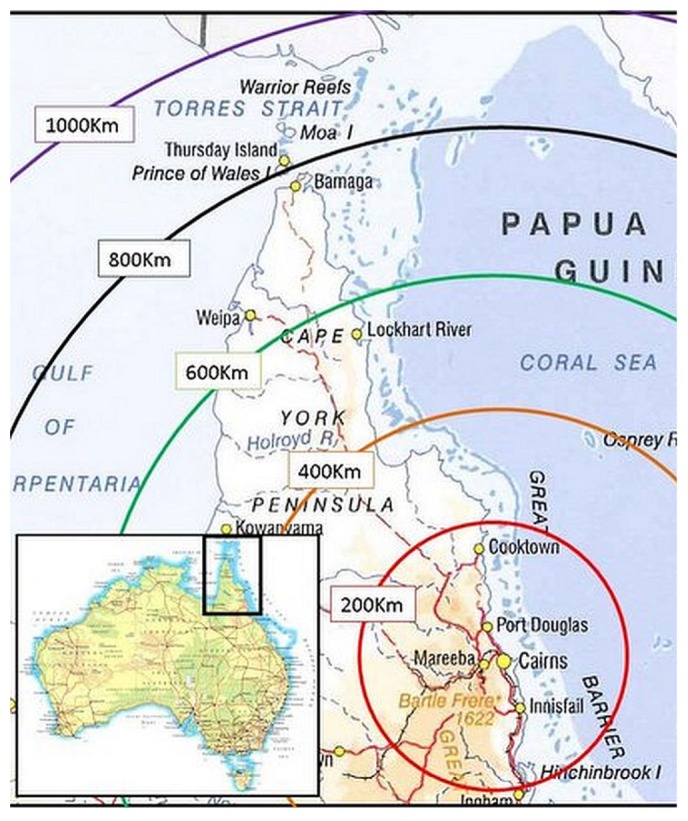
Area map of Far North Queensland, Australia.
In January 2003, the model of PD care was modified to achieve a considerable degree of ownership by the nephrology team. The model requires insertion of a PD catheter [Y-Tec peritoneoscope (Medigroup, Naperville, IL, USA) technique] by a nephrologist or advanced nephrology trainee (in the 2nd- or 3rd-year fellowship program); location of a procedure room adjacent to the PD training facility; involvement of a PD nurse as an assistant during the procedure and subsequent recovery of the patient; provision of hostel accommodation within walking distance of the hospital for patients and their carers for the duration of PD training; an indigenous liaison officer to coordinate with home and community care agencies to facilitate provision of a table, fly screens, air conditioning, electrical outlet, and so on; a home visit by the PD nurse on 3 occasions during the first year (1 week after the patient’s return to the community, and at 6 months and 12 months) and provision of education and unit protocols to the health workers in the primary health care units; and review by a nephrologist 4 times annually, either in-center or during outreach visits to remote communities.
The aim of the present study was to describe the outcome of the new model in relation to the timing of catheter placement, early procedural complications, and catheter survival in ATSI patients with ESKD from Far North Queensland, Australia.
Methods
This retrospective study was conducted at the renal unit, Cairns Base Hospital, Cairns, Australia. Data were collected on all ATSI patients having catheter implantation using the Y-Tec peritoneoscopic technique between 1 January 2003 and 31 July 2010.
Medical charts of all identified patients were individually reviewed. The recorded baseline data included demographics; primary renal disease; late referral (that is, commencement of PD within 30 days of first review by a nephrologist); catheter-related information, including insertion dates, commencement of PD, and early complications (PD peritonitis, leaks, and catheter malfunction occurring within 30 days of catheter insertion); period from catheter insertion to relocation to residence; and distance to residence. Catheter survival was defined as the time between insertion and removal of the catheter. If the patient received a replacement catheter, the second catheter was analyzed as a secondary event. The implantation technique did not change. The study was approved by the hospital ethics committee.
The patients were provided with culturally appropriate education about PD, often with the help of an indigenous health worker or liaison officer using written or pictorial aids. Informed consent was obtained before the procedure. Bowel preparation was used 12 - 24 hours before catheter insertion. Patients were given a topical antibacterial solution (Microshield T Triclosan Skin Cleanser: Johnson and Johnson Medical, North Ryde, Australia) to be applied on the entire abdominal wall the night before and the morning of the procedure. Patients received a prophylactic antibiotic (intravenous vancomycin or cephazolin) 1 hour before the procedure and an oral anxiolytic agent (diazepam 5 - 10 mg) 30 minutes before the procedure. Intravenous conscious sedation with midazolam and fentanyl was used if needed for patient comfort. However, all patients were kept alert to control their own respiratory pattern and to cooperate with requests to tense their abdominal musculature when required.
The standard technique for catheter implantation using a Y-Tec peritoneoscope was followed as described in the literature (4). Catheter implantation was performed by a staff nephrologist (MM) between 2003 and 2007. Subsequently, supervised catheter implantation was undertaken by the nephrology registrars (JK, RB) during their 2nd- or 3rd-year training; those individuals later joined the unit as staff nephrologists. In 2008, to minimize the risk of bowel trauma, a minor procedural modification was made, in which a Veress needle and air insufflation of the peritoneal cavity were applied before insertion of a preassembled trocar and quill sheath (5).
Statistical Analysis: Results are expressed as frequencies and percentages, mean ± standard deviation, or median with 25th - 75th percentile, as appropriate. A Kaplan-Meier curve was used to assess catheter survival censored for spontaneous recovery of renal function, transplantation, and death. Multivariate logistic regression analysis was performed to identify predictors of early complications (for example, primary catheter malfunction). Sex, presence of diabetes mellitus, body mass index (BMI), late referral, and age at the time of catheter insertion were included in the model as covariates. Any p values less than 0.05 were considered statistically significant. Data were analyzed using the software package PASW Statistics for Windows (release 18.0: SPSS, North Sydney, Australia).
Results
Table 1 shows that 92 catheters were inserted in 75 patients. Mean age was 52 years, and diabetic nephropathy was the most common cause of renal failure (73.3%). Median time from referral to catheter insertion was 10 days (interquartile range: 10 - 11 days). Mean BMI was 28.25 ± 6.4 kg/m2. Late referral to nephrology occurred in 36 patients (48%). The catheters used included the swan-neck straight (20.7%), straight (29.3%), and coiled (50.0%) types. Most insertions were day procedures (55.4%). Median time from insertion to PD commencement was 10 days (interquartile range: 2 - 11 days). Of the 92 catheters, 21 (22.8%) were able to be used for PD soon after insertion. The residential location of 60% of the patients was more than 200 km from the treatment facility.
TABLE 1.
Baseline Characteristics of Patients and Catheters
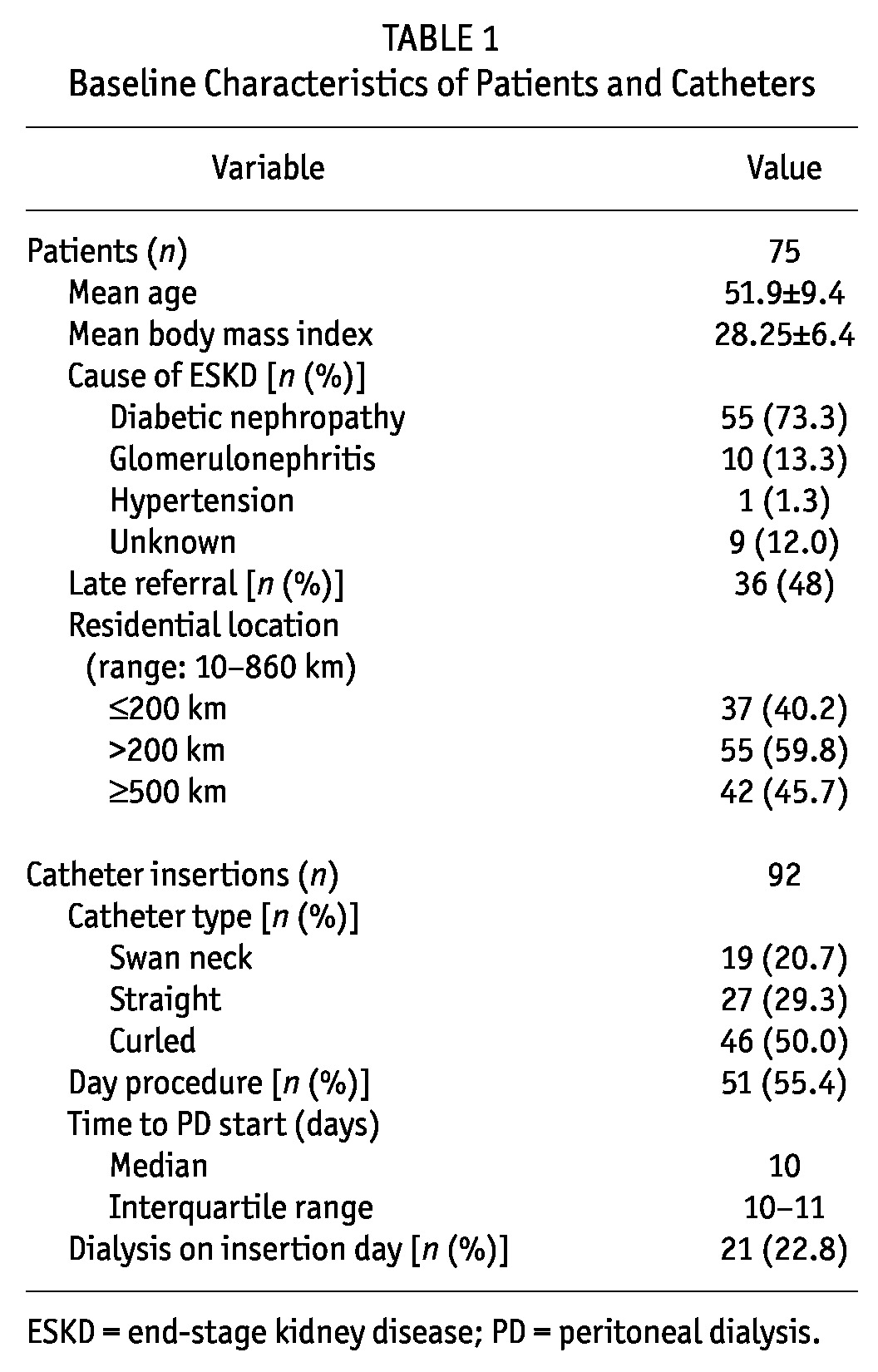
Table 2 shows that early complications occurred in 23.9% of catheters, including leaks (3.3%), exit-site infection (2.2%), peritonitis (3.3%), pericatheter bleeding (1.1%), and primary catheter malfunction (14.1%). Of the 13 catheters with primary malfunction, the causes were catheter migration (6 catheters), poor drainage (5 catheters), and extraperitoneal placement (2 catheters). Those catheters were removed and reinserted using the Y-Tec peritoneoscopic technique.
TABLE 2.
Early Catheter Complications
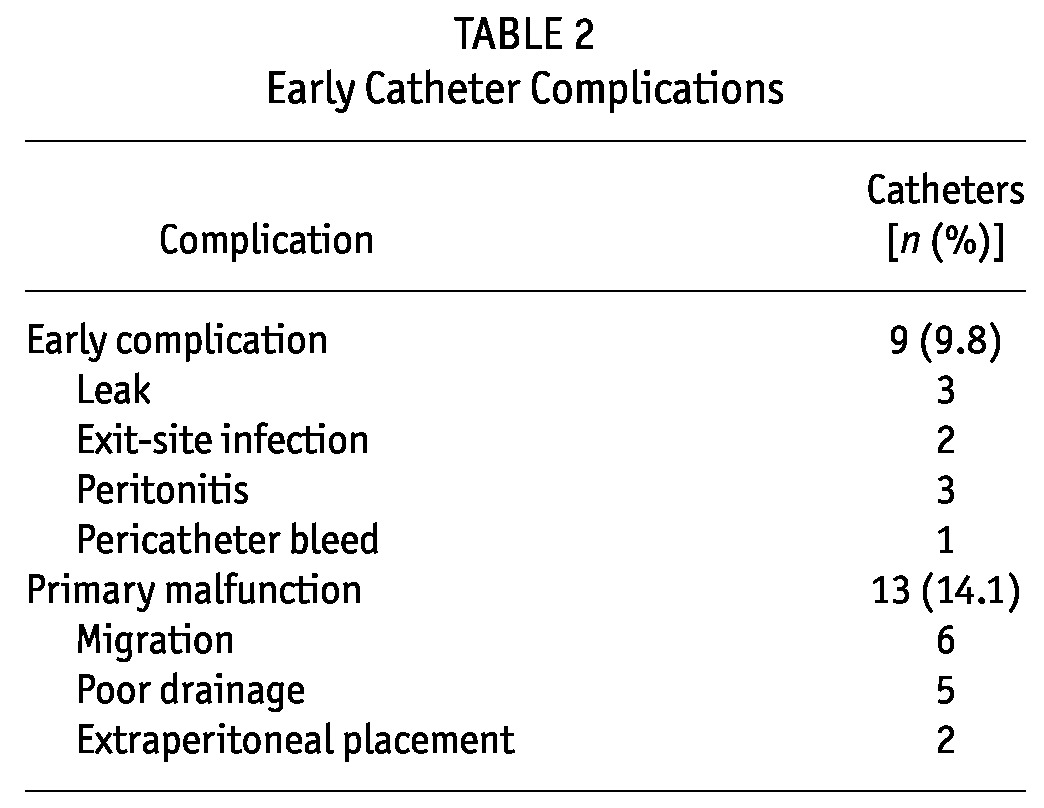
Table 3 lists reasons for the removal of an additional 27 catheters (after exclusions for transplantation and patient death with a functioning catheter). Those reasons were recurrent peritonitis (20 patients), scrotal leakage (1 patient), migration (4 patients), and omental wrap (2 patients). Primary catheter malfunction was not significantly predicted by a patient’s diabetes status [odds ratio (OR): 0.93; 95% confidence interval (CI): 0.14 to 6.08; p = 0.9], BMI (OR: 1.08; 95% CI: 0.96 to 1.20; p = 0.9), age (OR: 0.99; 95% CI: 0.90 to 1.08; p = 0.8), sex (OR: 0.98; 95% CI: 0.19 to 5.05; p = 0.9), late referral (OR: 3.14; 95% CI: 0.63 to 15.73; p = 0.2), or hospital admission for catheter insertion (OR: 0.65; 95% CI: 0.14 to 3.11; p = 0.6).
TABLE 3.
Reasons for Catheter Removala in 40 Patients
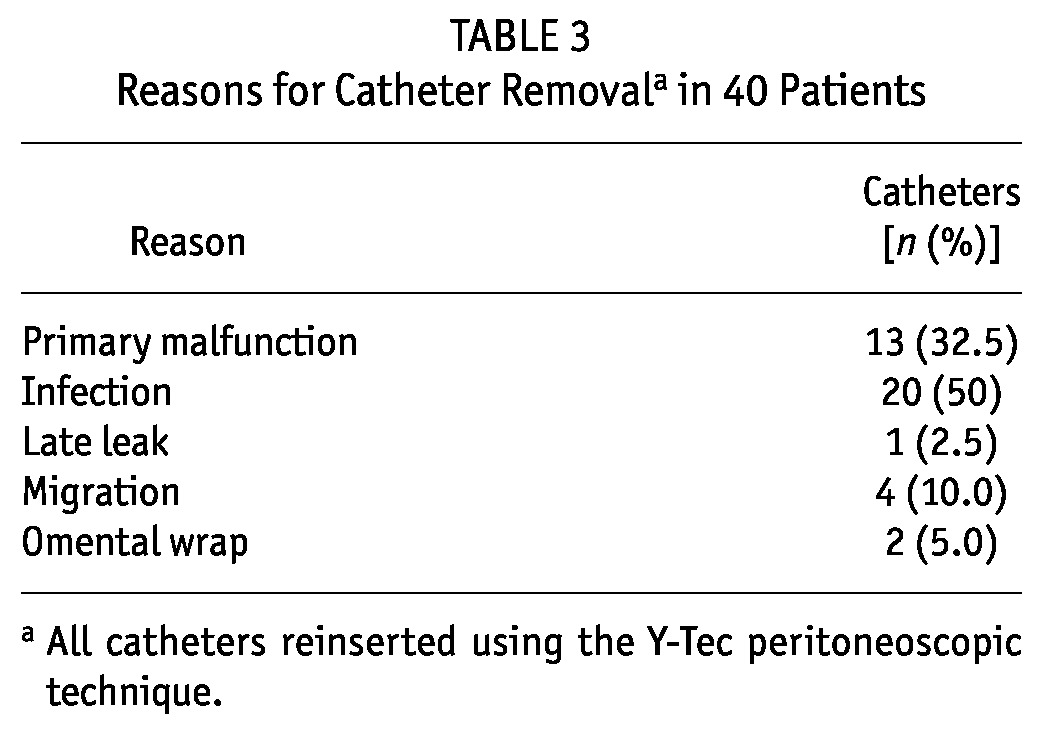
Figure 2 shows that the 12-month catheter survival was 79.2% and that the median technique survival was 23.3 months (95% CI: 8.7 to 38.5 months), censored for transplantation and death. Most of the patients (67 of 75, or 89%) were treated with automated PD.
Figure 2 —
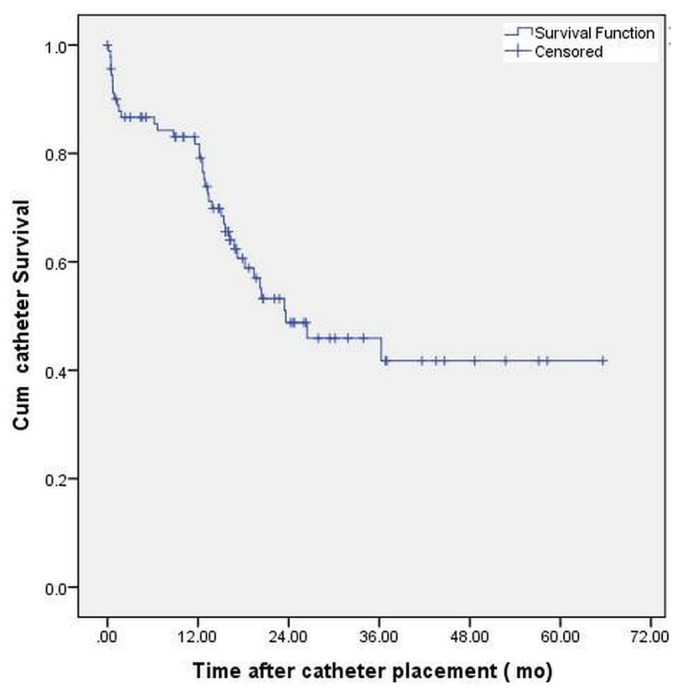
Kaplan-Meier curve demonstrating overall catheter survival in patients after Y-Tec peritoneoscopic insertion. Median catheter survival was 23.6 months.
Discussion
The International Society for Peritoneal Dialysis clinical practice guidelines for peritoneal access recommend having a center-specific dedicated team involved in the implantation and care of the catheter (6). The team should ideally consist of nephrologists, surgeons, and nurses. However, not all dialysis centers have a committed surgical team with a keen interest in peritoneal catheter placement (7). In that context, acquisition of catheter insertion skills by nephrologists makes intuitive sense. A large body of evidence suggests that, in the hands of an experienced nephrologist, a minimally invasive and safe technique such as the percutaneous Seldinger (8), fluoroscopically guided (9), or Y-Tec peritoneoscopic method (10) results in short-term and long-term catheter outcomes comparable to those with traditional surgery. Major advantages of those techniques are minimal lead time and lack of reliance on external specialties.
The relevance of the foregoing advantages is understated considering the burden of ESKD and the geographic isolation experienced by ATSI patients. Nearly 46% of our study population had to travel in excess of 500 km to the specialist facility, and the residential locales for almost all the patients were accessible by air travel only. No public transport to those areas is available by road, and roads are, in any case, are often inaccessible during the tropical wet season. Having the composition of the PD team and the procedural facility within the nephrology domain allowed for the implantation of PD catheters in a timely fashion (median: 10 days). Most patients (55%) could be returned to hostel accommodation or to the care of relatives in town after a satisfactory observation period of 4 hours post-procedure. In a study involving the Y-Tec implantation technique in a predominantly white population from a regional town in Australia, the average stay was 1.1 days (11).
The proximity of hostel accommodation to the PD unit allowed for close supervision and follow-up by the PD nurse, such that median time to commencement of PD training was 10 days (interquartile range: 10 - 11 days). Because of an established rapport and communication channel with remote primary health care facilities, a home care agency, and dialysis vendors (to ensure the presence of stocks of PD fluid and consumables ahead of the intended date of the patient’s return home), 45 patients (60%) were able to return to their place of residence in an average of 33.9 ± 5.9 days.
Because of the limited dissection of the anterior abdominal wall, peritoneoscopic implantation permitted immediate PD start with 21 catheters (22.8%), 1 of which had pericatheter leakage. The rates of early complication such as leaks (3.3%), exit-site infection (2.2%), and peritonitis (3.3%) encountered in the present study compare favorably with the results of the peritoneoscopic technique in the randomized controlled trial of Gadallah et al. (12). However, primary catheter malfunction occurred in 13 of the catheters (14.1%) implanted in our cohort, which is almost double the 7.9% reported by Gadallah et al. (12). Despite successful treatment of constipation and normal catheter placement (determined by computed tomography imaging of abdomen), we were unable to determine the cause of poor drainage. We surmised that kinks might be responsible. We did not attempt catheter manipulation; the catheters were removed and replaced with new catheters in one procedure.
We attempted catheter insertion in all dialysis-naive patients regardless of BMI, abdominal apron, or previous abdominal surgery. Nearly 50% of our cohort were either overweight (BMI: 25 - 29.9 kg/m2) or obese (BMI: ≥30 kg/m2). A multivariate logistic regression analysis showed that primary catheter malfunction could not be predicted from BMI. That finding can perhaps be attributed to the shortcomings of BMI as a measure of central adiposity, which is often better represented by waist-to-hip ratio. It is therefore still possible that those factors could have played a role. Indeed, customization of catheter selection and measurement of anthropometrics have been shown to improve outcomes of peritoneal access (13). The importance to indigenous patients of proximity to family and community support often plays a crucial role in their attitudes toward ESKD treatment options (14,15). In our experience, it is not uncommon for ATSI patients to decline in-center hemodialysis, a reflection of an overpowering desire to remain in their community. That choice is likely to limit the process of patient selection for the procedure.
Peritoneal infection (50%) was the leading cause of catheter removal contributing to technique failure. The ANZDATA Registry recently reported a 78% risk of peritonitis in Australian indigenous PD patients independent of demographic and comorbidity factors—perhaps a result of suboptimal personal and community cleanliness coupled with overcrowding and nonfunctional basic housing infrastructure (1).
Our 12-month catheter survival of 79.2% was similar to that reported in the literature (7,12). More than 90% of all PD catheters in our unit are inserted by a nephrologist.
Conclusions
In indigenous patients with ESKD, a close-knit model of PD delivery, with components including nephrologist-inserted catheters and integral involvement of PD nurses, can facilitate the provision of timely, safe, and efficient PD access, despite limitations of geographic remoteness. The incorporation of local health practitioners, indigenous allied health workers, and regular home visits by PD staff can also help to maintain therapy and prevent social dislocation.
Disclosures
The authors have no competing financial interests to declare.
References
- 1. Lim WH, Boudville N, McDonald SP, Gorham G, Johnson DW, Jose M. Remote indigenous peritoneal dialysis patients have higher risk of peritonitis, technique failure, all-cause and peritonitis-related mortality. Nephrol Dial Transplant 2011; 26:3366–72 [DOI] [PubMed] [Google Scholar]
- 2. Australian Bureau of Statistics (ABS). National Regional Profile 2007-2011 [Web resource]. Far North Queensland. Canberra, Australia: ABS; 2013. [Available at: http://www.ausstats.abs.gov.au/ausstats/nrpmaps.nsf/NEW+GmapPages/national+regional+profile?opendocument (search for “Far north (SA3) [31501] (QLD)”); accessed 22 October 2013] [Google Scholar]
- 3. Asif A, Byers P, Gadalean F, Roth D. Peritoneal dialysis underutilization: the impact of an interventional nephrology peritoneal dialysis access program. Semin Dial 2003; 16:266–71 [DOI] [PubMed] [Google Scholar]
- 4. Ash SR. Chronic peritoneal dialysis catheters: procedure for placement, maintenance and removal. Semin Nephrol 2002; 22:221–36 [DOI] [PubMed] [Google Scholar]
- 5. Asif A, Tawakol J, Khan T, Vieira CF, Byers P, Gadalean F, et al. Modification of the peritoneoscopic technique of peritoneal dialysis catheter insertion: experience of an interventional nephrology program. Semin Dial 2004; 17:171–3 [DOI] [PubMed] [Google Scholar]
- 6. Figueiredo A, Goh BL, Jenkins S, Johnson DW, Mactier R, Ramalakshmi S, et al. Clinical practice guidelines for peritoneal access. Perit Dial Int 2010; 30:424–9 [DOI] [PubMed] [Google Scholar]
- 7. Li PK, Chow KM. Importance of peritoneal dialysis catheter insertion by nephrologists: practice makes perfect. Nephrol Dial Transplant 2009; 24:3274–6 [DOI] [PubMed] [Google Scholar]
- 8. Henderson S, Brown E, Levy J. Safety and efficacy of percutaneous insertion of peritoneal dialysis catheter under sedation and local anesthesia. Nephrol Dial Transplant 2009; 24:3499–504 [DOI] [PubMed] [Google Scholar]
- 9. Moon JY, Song S, Jung KH, Park M, Lee SH, Ihm CG, et al. Fluoroscopically guided peritoneal dialysis catheter placement: long-term results from a single center. Perit Dial Int 2008; 28:163–9 [PubMed] [Google Scholar]
- 10. Ash SR, Wolf GC, Block R. Placement of Tenckhoff peritoneal dialysis catheter under peritoneoscopic visualization. Dial Transplant 1981; 10:382–6 [Google Scholar]
- 11. Kelly J, McNamara K, May S. Peritoneoscopic peritoneal dialysis catheter insertion. Nephrology (Carlton) 2003; 8:315–17 [DOI] [PubMed] [Google Scholar]
- 12. Gadallah MF, Pervez A, el-Shahawy MA, Sorrells D, Zibari G, McDonald J, et al. Peritoneoscopic versus surgical placement of peritoneal dialysis catheters: a prospective randomized study on outcome. Am J Kidney Dis 1999; 33:118–22 [DOI] [PubMed] [Google Scholar]
- 13. Crabtree JH, Burchette RJ, Siddiqi NA. Optimal peritoneal dialysis catheter type and exit site location: an anthropometric analysis. ASAIO J 2005; 51:743–7 [DOI] [PubMed] [Google Scholar]
- 14. Anderson K, Devitt J, Cunningham J, Preece C, Cass A. “All they said was my kidneys were dead”: indigenous Australian patients’ understanding of their chronic kidney disease. Med J Aust 2008; 189:499–503 [DOI] [PubMed] [Google Scholar]
- 15. Wilson R, Krefting L, Sutcliffe P, Van Bussel L. Native Canadians relocating for renal dialysis. Psychosocial and cultural issues. Can Fam Physician 1994; 40:1934–41 [PMC free article] [PubMed] [Google Scholar]


