Figure 1. Injectable polymer microgel and in vivo peptide release.
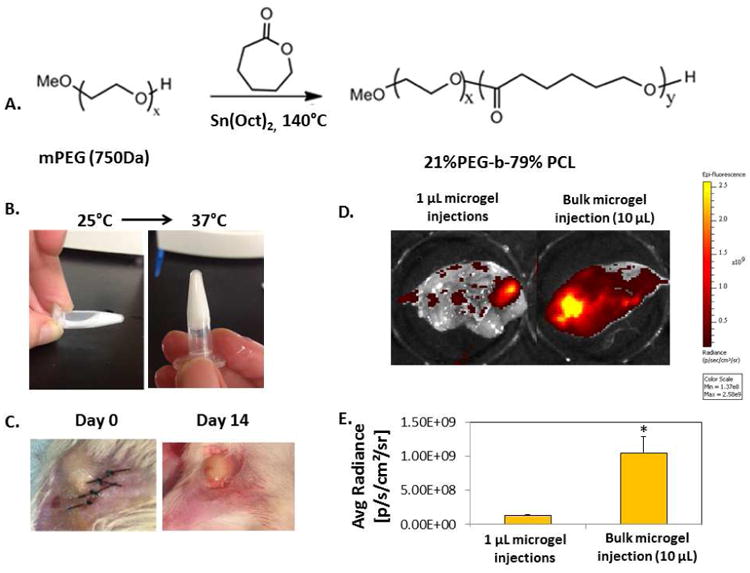
A) A co-polymer of 21% polyetheylene glycol (PEG) and 79% poly-ε-caprolactone (PCL) (%: molar ratio) was synthesized by ring opening polymerization with tin (II) ethyl hexanoate (Sn(Oct)2) catalyst. B) The injectable polymer microgel was a liquid at room temperature (25°C, left) and formed a stable gel at body temperature (37°C, right). C) Injectable polymer microgel immediately after surgery (day 0, left), or after 14 days (right). D) FITC-tagged Ac-SDKP was mixed with polymer microgel at 25°C before injecting either a single bulk 10 μL injection (right), or ten individual 1 μL injections into the muscle around the site of femoral artery ligations. After 7 days, the skin was removed from the thigh muscle and imaged using a fluorescence in vivo imaging system (IVIS). Areas with high fluorescent intensity are colored yellowed in the images. E) Fluorescence intensity of peptides that remained in the tissue after 7 days was quantified using IVIS software. As evidenced by the lower fluorescence intensity of peptides retained in the tissue, more of the peptides were released with multiple 1 μL injections than with the bulk injection over the course of 7 days. D-E) n=4; Data are means ± SEM from four or five mice. *p<0.05 vs. 1 μL polymer microgel injections (Tukeys' Range Test).
