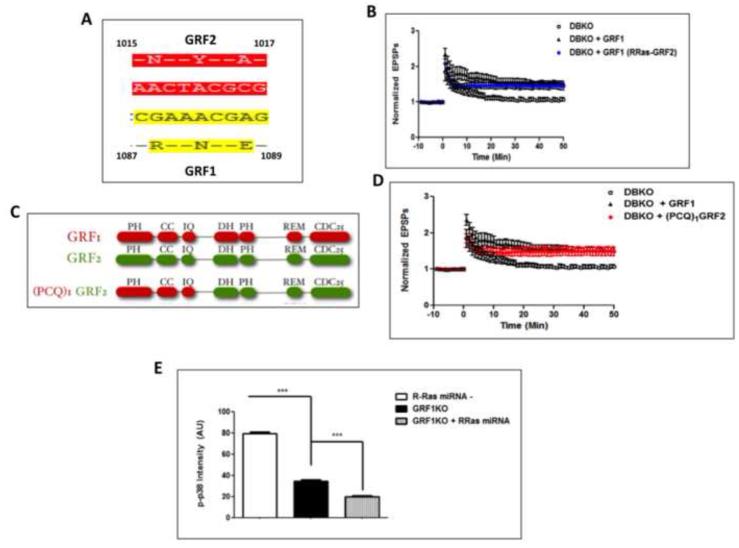
Figure 5. GRF1 induces HFS-LTP in an R-Ras-independent manner and R-Ras acts independently of GRF1 in promoting HFS-induced p38 activation.
A. The amino acid differences in the CDC25 domains between GRF1 and GRF2 responsible for activation of R-Ras by GRF1 (Gotoh et al., 2001) B. HFS-LTP in brain slices from double GRF1/GRF2 knockout (DBKO) mice and DBKO mice injected with adenovirus expressing wild-type GRF1 or the GRF1/(R)GRF2 chimera that can no longer activate R-Ras into the CA1 hippocampus (DBKO 111 ± 4.3% ; WT-GRF1, 165 ± 9.4%; GRF1/(R-Ras-GRF2, 148 ± 8.5%, Two-Way ANOVA, Virus effect, F 2,944 = 7.76, p = 0.0044; Bonferroni post-hoc, WT-GRF1 vs. GRF1/(R-Ras-GRF2), p > 0.05 at all time points). C. Functional domains in chimera that replaces the whole C-terminus of GRF1 with that of GRF2, including the CDC25 domain of GRF1 that is required for R-Ras activation. (PH- pleckstrin homology, CC- coiled-coil, IQ- calmodulin binding domain, DH- Dbl homology, REM- Ras exchange motif, CDC25- Ras and R-Ras activating domain) D. HFS-LTP in brain slices of DBKO mice is reconstituted in the CA1 with (PCQ)1GRF2. (DBKO, 111.63 ± 2.23%; WT-GRF1, 165 ± 9.4%; (PCQ)1GRF2, 150 ± 10.7%, Two-Way ANOVA, Virus effect, F2,944 = 6.06, p = 0.011; Bonferroni post-hoc, WT-GRF1 vs. (PCQ)1GRF2, p > 0.05 at all time points, also see data as reported in (Jin et al., 2014, Fig. 3A) E. Hippocampal slices from mice previously injected with virus expressing R-Ras miRNA1 or 2 into the CA1 area of RasGRF1 KO mice were stimulated with HFS-LTP and processed for immunostaining of phosphorylated p38. A comparison of levels of phosphorylated p38 immunostaining between wild-type cells (WT), GRF1 KO cells and GRF1 KO cells containing R-Ras miRNAs revealed an additive effect on p-p38 inhibition by R-Ras in cells already lacking RasGRF1 (One-Way ANOVA, F 2,535 = 817.5, p < 0.0001, Bonferroni post-hoc: R-Ras miRNA− vs. GRF1-KO, t = 19.23, p < 0.05; GRF1-KO vs. GRF1-KO+R-Ras miRNA, t = 5.95, p < 0.05).