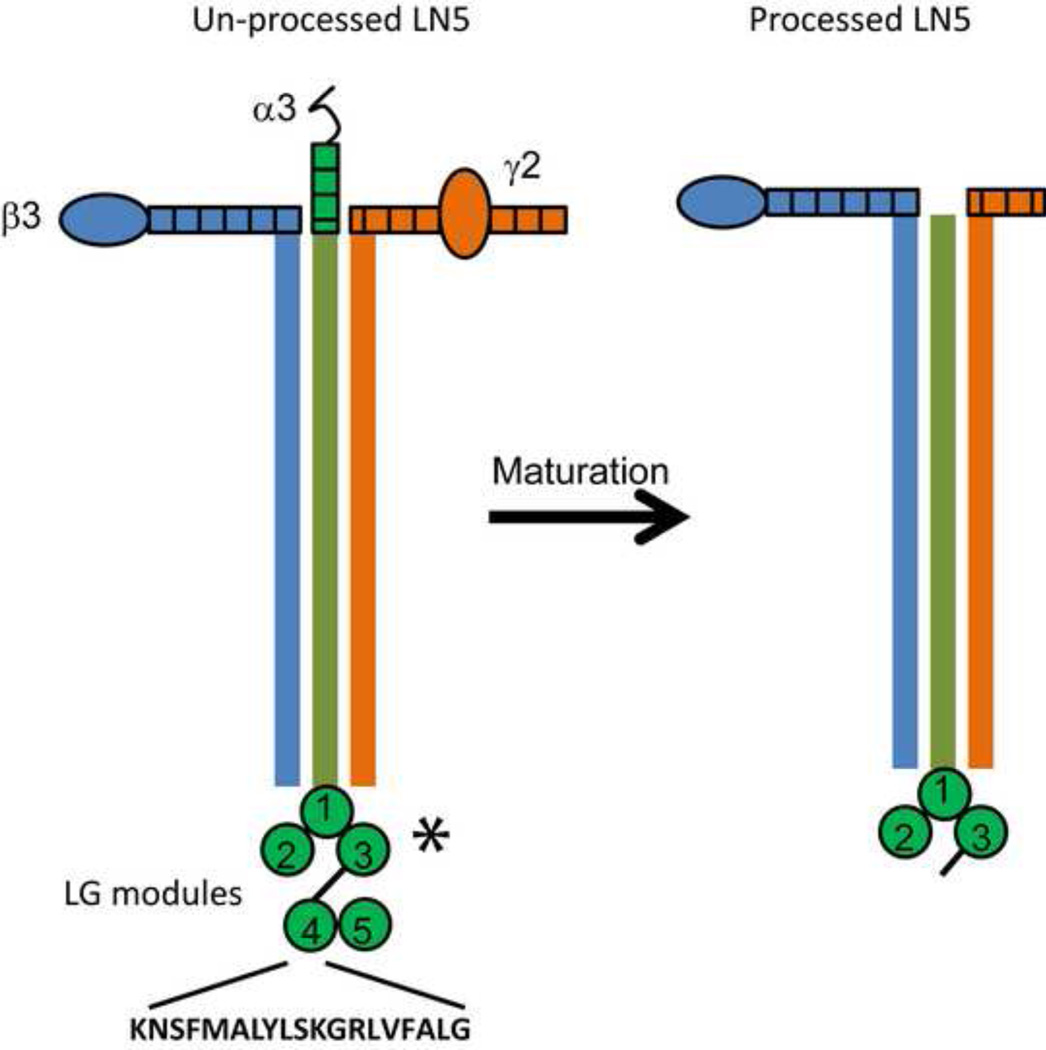
Figure 4. Schematic of laminin 5.
Laminin 5 (laminin 332) is a heterotrimeric, multi-domain-containing ECM protein that is composed of α3, β3 and γ2 subunits. The unprocessed form of the protein contains a large globular domain at the C-terminal end of the α3 chain which is composed of five LG modules (LG1-5). The binding sites for α3β1, a6β1 and α6β4 integrins are located within the first three LG modules (asterisk) while the syndecan 4 binding PEP75 sequence (KNSFMALYLSKGRLVFALG) is located within the LG4 module. After secretion and deposition into the ECM, the protein undergoes proteolytic processing that results in the loss of N-terminal portions of both the α3 and γ2 chains as well as cleavage of the LG4 and LG5 modules from the C-terminus of the α3 chain. Thus the PEP75-containing module of laminin 5 is potentially free to interact with cell surface receptors such as syndecan 4 independent of the rest of the protein.