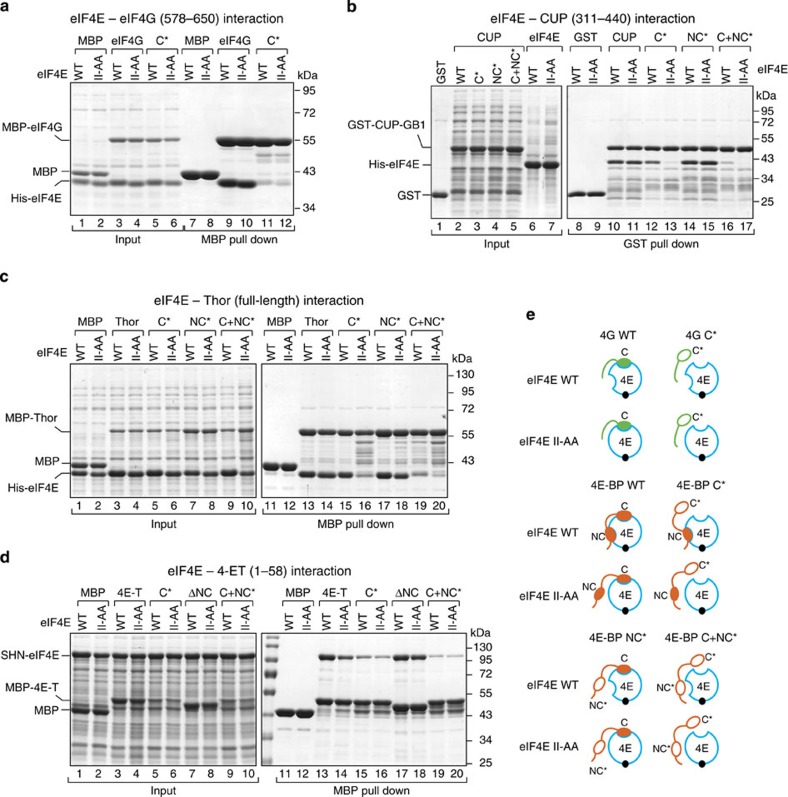
Figure 3. 4E-BPs interact with eIF4E using a bipartite-binding mechanism.
(a) MBP pull-down assay showing the interaction of His6-eIF4E (full length, either WT or II-AA mutant) and MBP-eIF4G (residues 578–650; either WT or canonical 4E-BM mutant (C*)). The input (25%) and bound fractions (50%) were analyzed by SDS–PAGE followed by Coomassie blue staining. (b) GST pull-down assay showing the interaction of His6-eIF4E (WT or II-AA mutant) and GST-CUP-GB1 (residues 311–440, WT or mutated in the canonical motif (C*), non-canonical motif (NC*) or both motifs (C+NC*)). (c) MBP pull down showing the association of MBP-Thor (full length, WT or 4E-BM mutants) with His6-eIF4E (WT or II-AA mutant). The samples were analyzed as described in a. (d) MBP pull-down assay showing the interaction of MBP–4E-T (fragment 1–58, WT or the indicated mutants) with eIF4E (WT or II-AA mutant). eIF4E was expressed with a tag consisting of the streptavidin-binding peptide (strep), His6 and the NusA protein (SHN tag). (e) Schematic representation of the different eIF4E–4E-BPs and eIF4E–eIF4G complexes that were analyzed in the pull-down assays. 4E, eIF4E (blue circle). Black circle, m7GTP-cap structure. The 4E-BPs are shown in orange and the eIF4G in green. The asterisks indicate mutations in the corresponding motifs. These mutations are described in Supplementary Table 1.