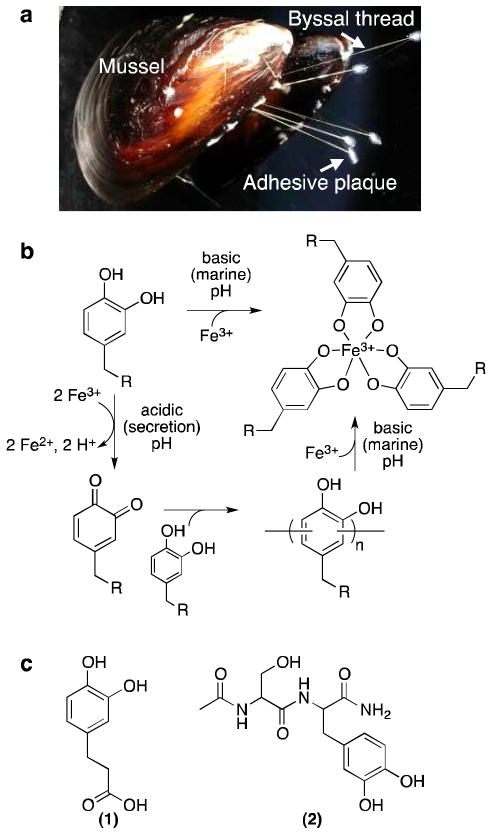
Figure 1.
Mussel byssus, model for pH-dependent Fe3+-catechol chemistry, and molecules studied. (a) Picture of mussel with labeled byssal structures. (b) Model for pH-dependent interactions between Fe3+ and catechol. At acidic pH, catechols and Fe3+ react to produce o-quinones, leading to catechol-catechol oligomerization. At basic pH, catechols form coordination bonds with Fe3+ that prevent oxidation of the catechol. In the context of the mussel byssus, Fe3+ could hypothetically induce covalent cross-linking during acidic processing of byssal protein secretions and enhance the mechanical properties of the byssal thread and adhesive by formation of mechanically active Fe3+-catechol coordination interactions upon equilibration to basic marine pH. Note that Fe3+-catechol coordination bonds would be expected to be mechanically reversible, whereas covalent catechol-catechol bonds would not.23 (c) Catechol-containing molecules studied: (1) DHPA and (2) Ac-Ser-DOPA-NH2 (dipeptide).