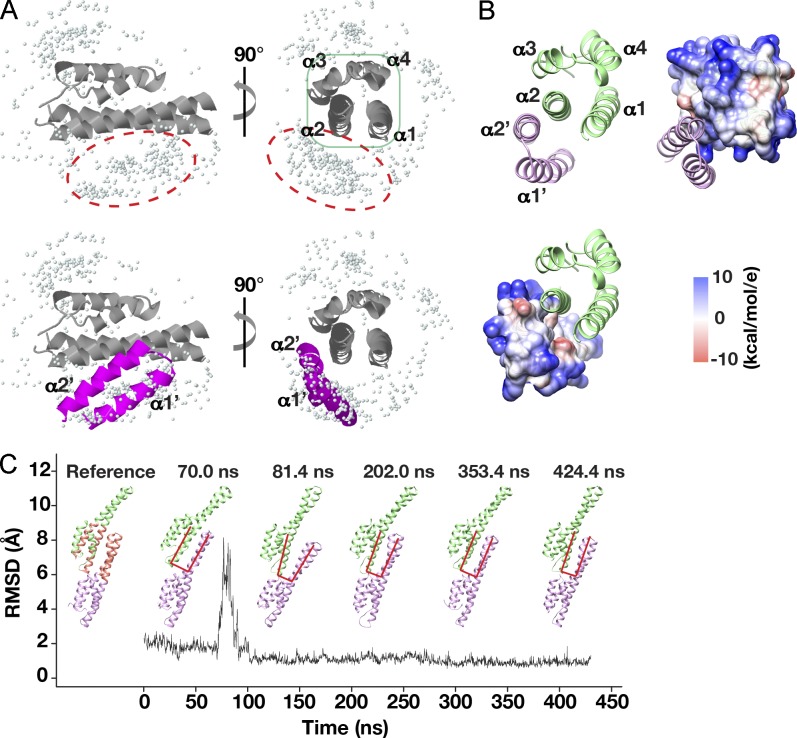
Figure 4.
Stable interactions between neighboring α2 helices drive hVps24 dimer formation. (A) The ZDOCK server was used to determine the relative positions of the helical hairpin from one Vps24 monomer and the asymmetric four-helix bundle of a neighboring Vps24 subunit, as described in Fig. 3 C. (B) The human Vps24 dimer interface is dominated by hydrophobic interactions (white), with additional contributions by positively and negatively charged amino acids in the neighboring α2 helices (as described for Vps32). (C) Calculations of the RMSD of the α carbon atoms in the hVps24 dimer interface, relative to a reference structure (left), using an explicit solvent simulation that demonstrates its stability. Representative snapshots of the structure at different time points are shown as described for Fig. 3 E. The black line indicates the RMSD relative to the time of the simulation.