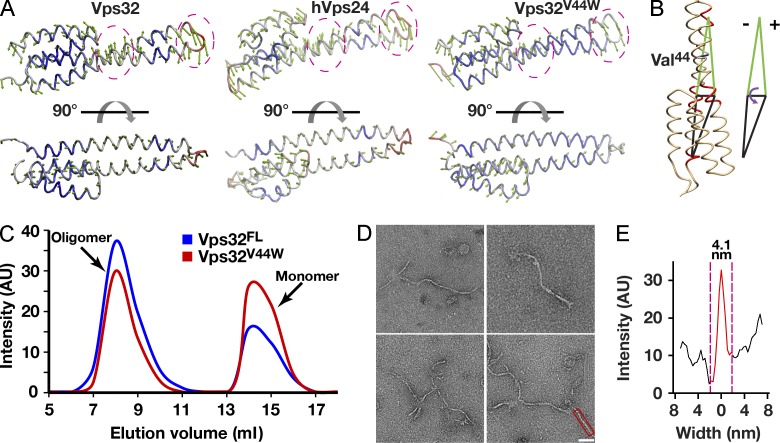
Figure 6.
Increasing the rigidity of the Vps32 helical hairpin inhibits spiral filament assembly. (A) Principal component analysis was used to compare the flexibilities of wild-type C. elegans Vps32, human Vps24, and a mutant isoform of C. elegans Vps32 (Vps32V44W), predicted to exhibit a more rigid architecture. Green arrows indicate the direction of movement, and the length of the arrows indicates the magnitude of motion. The degree of structural flexibility is highlighted with different colors, with red indicating regions of high flexibility and blue indicating regions of low flexibility. The hinge region and the tip of the helical hairpin are circled for comparison. (B) The bending angle can be defined as the change in a specific dihedral angle in Vps32. The dihedral is composed of the four geometric centers of α carbon atoms in three motifs (residues 18–21, 29–33, and 52–55) in helix α1 and a single motif (residues 79–82) in helix α2. The position of valine 44 is highlighted. (C) A comparison of the elution profiles of wild-type (FL, full length) and mutant (V44W) Vps32 after gel filtration chromatography indicates only minor differences in their hydrodynamic properties. The data shown are from single representative experiments, which were repeated independently at least two times for each protein. (D) Representative negatively stained EM images of purified, recombinant Vps32V44W, which continues to form filaments but fails to assemble into spirals or ring structures. Bar, 40 nm. (E) Width analysis of a Vps32V44W filament that is highlighted in D (intensity after background subtraction was used to calculate the width of filaments). The data shown are from a single representative experiment, which was repeated independently three times (n = 15 for the experiment shown). AU, arbitrary unit.