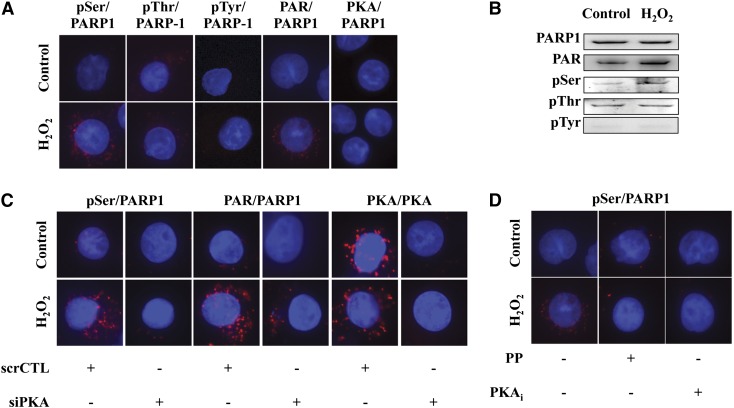
Fig. 9.
PARP1 is specifically phosphorylated by PKA on serine residues in U937 cells during oxidative stress. (A) Serine-specific phosphorylation of PARP1, as evidenced by in situ PLA analysis. Control experiments show the auto-PARylation of PARP1. (B) H2O2 induces serine-specific (but not threonine- and tyrosine-specific) phosphorylation of PARP1. (C) Serine-specific phosphorylation of PARP1 is reduced by propranolol (PP) and PKAi in H2O2-treated cells. (D) PKA silencing reduces the phosphorylation of PARP1 on serine residues and attenuates the level of PARP1 auto-PARylation. Figures show representative images of at least n = 3 independent determinations conducted on different experimental days.