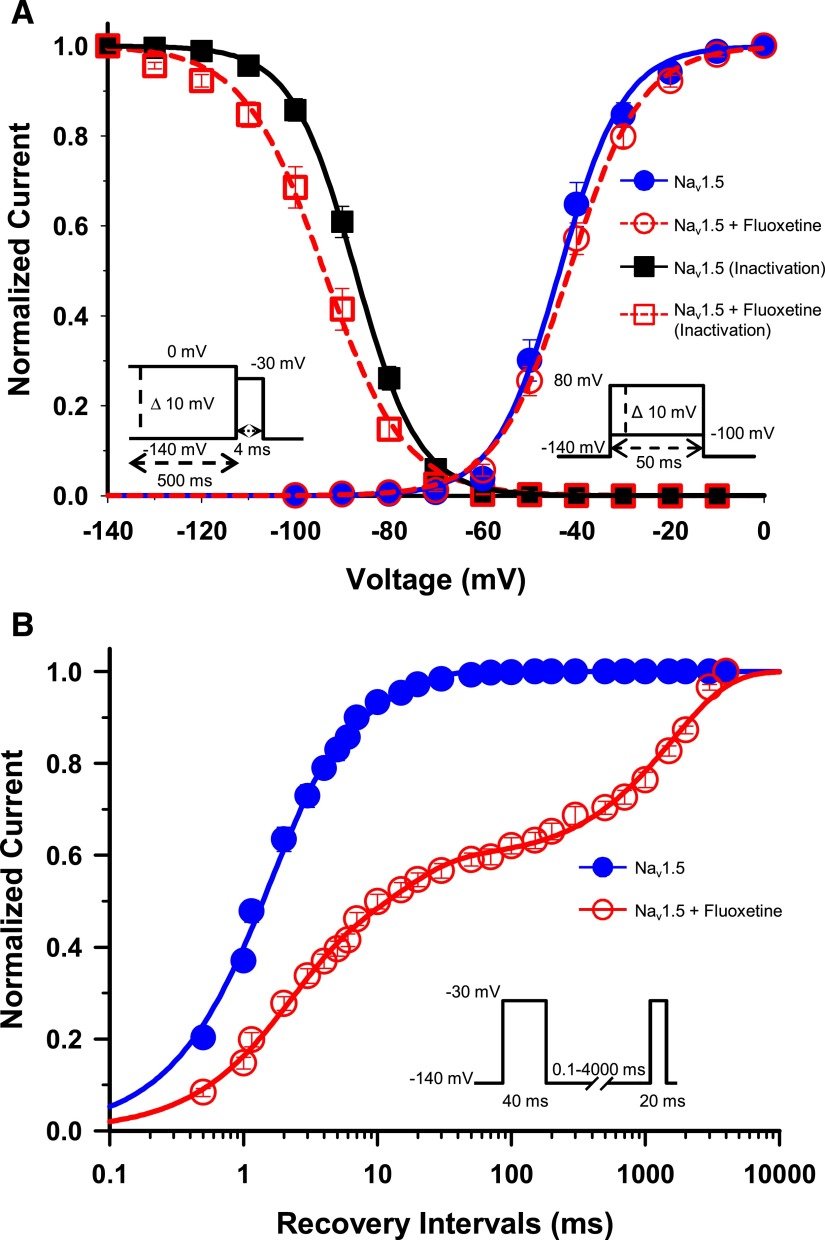
Fig. 2.
Gating properties of Nav1.5/WT treated with fluoxetine. (A) Voltage dependence of steady-state activation and inactivation of Nav1.5. Cells were perfused with external solution as a control (activation, n = 14; inactivation, n = 19) or with 30 µM racemic fluoxetine (activation, n = 18; inactivation, n = 17). Activation curves were elicited with 50-millisecond depolarizing steps from −100 to 80 mV in 10 mV increments. Cells were held at a holding potential of −140 mV. Fluoxetine caused no significant shift in the activation curve. Steady-state inactivation was determined using 4-millisecond test pulses to −30 mV after a 500-millisecond prepulse to potentials ranging from −140 mV to 0 mV (see the inset under the inactivation curves for the protocol). The application of 30 µM fluoxetine induced a significant −6.7 mV shift of the inactivation curve (Table 1). The activation and inactivation curves were fitted to a single Boltzman function (see Materials and Methods). (B) Recovery from inactivation of Nav1.5 in the absence (n = 10) or presence (n = 9) of 30 µM fluoxetine. The cells were depolarized to −30 mV for 40 milliseconds from a holding potential of −140 mV to inactivate all the Na+ channels. Test pulses were then applied to −30 mV for 20 milliseconds to measure current amplitudes, with an interval ranging from 0.1 to 4000 milliseconds. The resulting curves were fitted to a double (control) or a triple (+ fluoxetine) exponential equation, which yielded two or three time constants (τ1, τ2, τ3). The application of 30 µM fluoxetine strongly slowed the recovery from inactivation with the appearance of a third time constant (Table 1).