Abstract
Direct activation of the endocannabinoid receptor G protein–coupled receptor 18 (GPR18) in the rostral ventrolateral medulla (RVLM) of conscious rats by abnormal cannabidiol (Abn CBD; trans-4-[3-methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]-5-pentyl-1,3-benzenediol) elevates local nitric oxide (NO) and adiponectin (ADN) levels and reduces oxidative stress and blood pressure (BP). However, the molecular mechanisms for GPR18-mediated neurochemical responses, including the nitric oxide synthase isoform that generates NO, and their potential causal link to the BP reduction are not known. We hypothesized that GPR18-mediated enhancement of Akt, extracellular signal-regulated kinase 1/2 (ERK1/2), and neuronal nitric oxide synthase (nNOS) phosphorylation, triggered by a reduction in cAMP, accounts for the NO/ADN-dependent reductions in RVLM oxidative stress and BP. Intra-RVLM GPR18 activation (Abn CBD; 0.4 μg) enhanced RVLM Akt, ERK1/2, and nNOS phosphorylation as well as ADN levels during the hypotensive response. Prior GPR18 blockade with O-1918 (1,3-dimethoxy-5-methyl-2-[(1R,6R)-3-methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]benzene) produced the opposite effects and abrogated Abn CBD–evoked neurochemical and BP responses. Pharmacological inhibition of RVLM phosphoinositide 3-kinase (PI3K)/Akt (wortmannin), ERK1/2 (PD98059 [2-(2-amino-3-methoxyphenyl)-4H-1-benzopyran-4-one]), or nNOS (Nω-propyl-l-arginine), or activation of adenylyl cyclase (forskolin) virtually abolished intra-RVLM Abn CBD–evoked hypotension and the increases in Akt, ERK1/2, and nNOS phosphorylation and in ADN levels in the RVLM. Our pharmacological and neurochemical findings support a pivotal role for PI3K, Akt, ERK1/2, nNOS, and adenylyl cyclase, via modulation of NO, ADN, and cAMP levels, in GPR18 regulation of the RVLM redox state and BP in conscious rats.
Introduction
Several novel cannabinoid receptors, including G protein–coupled receptor 18 (GPR18), mediate diverse cardiovascular effects (Offertáler et al., 2003; Kohno et al., 2006; McHugh et al., 2010). Our recent findings (Penumarti and Abdel-Rahman, 2014) demonstrated GPR18 expression in rostral ventrolateral (RVLM) neurons, which modulate the sympathetic activity (Sved et al., 2003; Kishi et al., 2004; Guyenet, 2006), and suggested a restraining influence for RVLM GPR18 on blood pressure (BP). N-Arachidonoyl glycine (NAGly), a metabolite of the endocannabinoid anandamide, serves as an endogenous GPR18 agonist (McHugh et al., 2010). Furthermore, GPR18 activation by its synthetic agonist, abnormal cannabidiol (Abn CBD; trans-4-[3-methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]-5-pentyl-1,3-benzenediol), mediates cannabinoid receptor 1 (CB1R)– and cannabinoid receptor 2–independent responses, such as nitric oxide (NO)–dependent vasorelaxation (Offertáler et al., 2003; McHugh et al., 2010). However, it must be remembered that indirect activation of CB1R by NAGly, but not Abn CBD, influences the GPR18-mediated responses in tissues that contain both receptors, particularly in whole animal studies (Penumarti and Abdel-Rahman, 2014).
Reported signaling studies, mostly conducted in isolated tissues, show that GPR18 activation enhances phosphorylation of extracellular signal-regulated kinase 1/2 (ERK1/2) (Alexander, 2012) and Akt (McCollum et al., 2007). It is imperative to note, however, that there are no studies that have elucidated the roles of these kinases in central GPR18 signaling, particularly the nitric oxide synthase (NOS) isoform that mediates NO elevation in the RVLM and the potential causal role for these molecular events in the GPR18-mediated hypotension. This is important because the role of the phosphoinositide 3-kinase (PI3K)-Akt-ERK1/2 pathway in modulating the redox state in the RVLM and subsequently BP is controversial. Enhanced ERK1/2 phosphorylation and elevated NO levels in the RVLM mediate a pressor (Ibrahim and Abdel-Rahman, 2012a,b; Chan and Chan, 2014) or hypotensive (Zhang and Abdel-Rahman, 2005; Nassar and Abdel-Rahman, 2008; Chan and Chan, 2014) response. Furthermore, enhanced brainstem ERK1/2 phosphorylation by activation of angiotensin II receptor 1 leads to reactive oxygen species (ROS) generation and BP elevation (Kishi et al., 2004; Chan et al., 2007; Hirooka, 2008). Therefore, we considered the possibility that NO-dependent elevation of a downstream antioxidant or pro-oxidant molecule in the RVLM might determine the final redox state, and the ultimate BP response. In this regard, we reported preliminary “correlative” evidence that adiponectin (ADN), which reduces oxidative stress (Song et al., 2013), is a potential downstream mediator of the hypotension caused by RVLM GPR18 activation (Penumarti and Abdel-Rahman, 2014).
Here, we tested the hypothesis that GPR18 activation of the RVLM PI3K-Akt-ERK1/2-neuronal nitric oxide synthase (nNOS) network, triggered by reduction in cAMP, causes NO-dependent elevation in RVLM ADN levels, which ultimately reduces RVLM oxidative stress and BP. To test this hypothesis and establish a causal role for these molecular events in GPR18-mediated hypotension, we investigated the effects of pharmacological interventions that inhibit these molecular events or elevate the cAMP level in the RVLM in the absence or presence of local GPR18 activation in conscious unrestrained rats. Molecular studies conducted in RVLM tissues, collected from control and treatment groups at the conclusion of the cardiovascular studies, complemented the pharmacological findings and facilitated data interpretation.
Materials and Methods
Male Sprague-Dawley rats (Charles River Laboratories, Raleigh, NC), aged 11 to 12 weeks, were used in this study. After vascular catheterization and intra-RVLM cannulation, the rats were housed individually in separate cages in a room with a controlled environment. The temperature was maintained at 23°C ± 1°C, with 50% ± 10% humidity and a 12-hour light/dark cycle. Food (Prolab Rodent Chow; Granville Milling, Creedmoor, NC) and water were provided ad libitum. All surgical and experimental procedures are detailed in the Supplemental Materials and Methods and were conducted in accordance with and approved by the East Carolina University Institutional Animal Care and Use Committee and in accordance with the Guide for the Care and Use of Laboratory Animals (Institute for Laboratory Animal Research, 2011). These surgeries were performed as reported in our previous studies (Zhang and Abdel-Rahman, 2002; Penumarti and Abdel-Rahman, 2014). On the day of the experiment, the arterial catheter was flushed with heparin in saline (200 U/ml), connected to a Gould-Statham (Oxnard, CA) pressure transducer, and the BP of unrestrained rats was measured as mentioned in our previous studies (Nassar et al., 2011; Ibrahim and Abdel-Rahman, 2012a). Expanded Materials and Methods are available in the Supplemental Materials and Methods.
Experimental Groups and Protocol
Experiment 1: Effect of Intra-RVLM Inhibition of ERK1/2, PI3K/Akt, or NOS or Adenylyl Cyclase Activation on Abn CBD–Mediated Hypotensive Response.
The objective of conducting these and the complementary ex vivo molecular studies (see below) was to establish a causal role for the enhanced phosphorylation of RVLM Akt, ERK1/2, and nNOS in GPR18-mediated hypotension. Therefore, we investigated the BP and heart rate (HR) responses elicited by intra-RVLM Abn CBD in the absence or presence of pharmacologic inhibitors of PI3K/Akt (wortmannin; 100 nmol) (Seyedabadi et al., 2001), ERK1/2 (PD98059 [2-(2-amino-3-methoxyphenyl)-4H-1-benzopyran-4-one]; 50 μmol) (Alessi et al., 1995; Seyedabadi et al., 2001), endothelial nitric oxide synthase (eNOS) (L-NIO [N5-(1-iminoethyl)-l-ornithine]; 100 pmol) (El-Mas et al., 2009), or nNOS (NPLA [Nω-propyl-l-arginine hydrochloride]; 250 pmol) (El-Mas et al., 2009) based on reported studies. Dimethylsulfoxide (DMSO) was used as the vehicle for all kinase inhibitors, whereas saline was the vehicle for NOS inhibitors. DMSO was diluted in artificial cerebrospinal fluid (1:16) and this DMSO/artificial cerebrospinal fluid mixture had no effect on BP, which is consistent with our previous findings (Nassar et al., 2011). The rats in each group (n = 6 unless otherwise specified) received one of the following treatment combinations (80 nl): 1) DMSO plus vehicle or Abn CBD (n = 3 each), 2) saline plus vehicle or Abn CBD (n = 3 each), 3) wortmannin plus vehicle or Abn CBD, 4) PD98059 plus vehicle or Abn CBD, 5) L-NIO plus vehicle or Abn CBD, or 6) NPLA plus vehicle or Abn CBD. A pretreatment (DMSO, saline, wortmannin, PD98059, L-NIO, or NPLA) was microinjected into the RVLM 30 minutes before Abn CBD (0.4 μg) or vehicle; this dose was based on our recent findings (Penumarti and Abdel-Rahman, 2014). At the end of BP measurement (30 minutes after intra-RVLM Abn CBD or vehicle injection), the brains were collected after euthanasia and stored at −80°C for neurochemical measurements.
Experiment 2: Effect of Intra-RVLM Activation of Adenylyl Cyclase on Abn CBD–Mediated Hypotensive Response.
The objective of this experiment was to test the hypothesis that adenylyl cyclase (AC) inhibition (reduced cAMP level) in the RVLM contributes to GPR18-mediated neurochemical responses and hypotension because GPR18 is a Gi-coupled receptor (Kohno et al., 2006) and because activation of Gi-coupled receptors, such as the α2A-adrenergic receptor, which inhibits AC/cAMP in the brainstem, lowers BP (Tsuda et al., 2003). Since measurement of the cAMP level in the RVLM was not feasible in the scarce amount of RVLM tissue, we adopted an alternative approach that involves pharmacological activation of intra-RVLM AC by forskolin (50 μmol) microinjection (McHugh et al., 2010) in four groups of conscious male rats as follows: 1) DMSO plus vehicle or Abn CBD (n = 3 each), and 2) forskolin plus vehicle or Abn CBD (n = 6 each). Forskolin or its vehicle (DMSO) was microinjected 30 minutes before Abn CBD (0.4 μg) or its vehicle (methyl acetate). At the end of cardiovascular measurements (30 minutes after Abn CBD or vehicle injection), the rats were euthanized and brains were collected and stored at −80°C for biochemical measurements.
Experiment 3: Effect of Akt-ERK1/2-nNOS Inhibition or Increased cAMP on GPR18-Mediated Molecular Events in the RVLM.
Ex vivo biochemical studies were conducted in RVLM tissues collected from treatment and control animals utilized in the above-described integrative (in vivo) cardiovascular studies. We measured RVLM phosphorylated pERK1/2, pAKT, p-nNOS, ADN, and ROS levels in rats treated with intra-RVLM Abn CBD or vehicle in the absence or presence of inhibitors of ERK1/2 (PD98059), PI3K/Akt (wortmannin), or nNOS (NPLA), or activation of AC (forskolin) using dot blot analysis and measured the ROS level by the dichloro-dihydro-fluorescein diacetate assay as detailed in the Supplemental Materials and Methods.
Two additional groups of rats were used. In the first group (n = 5), we determined whether measurement of RVLM ADN and ROS levels in brains collected 15 minutes after intra-RVLM Abn CBD (0.4 μg), during the maximal hypotensive response, would uncover greater RVLM ADN elevation and lower ROS levels than those observed in rats euthanized 30 minutes after Abn CBD when the hypotensive response was dissipating. Immunohistochemical measurement of the ADN-immunoreactive neurons was conducted as detailed in the Supplemental Materials and Methods. In the second group (n = 4), we determined whether microinjection of a relatively low dose (0.5 pmol) of ADN into the RVLM reduces BP and RVLM ROS. In both groups, the neurochemical responses observed in the treated RVLM were compared with the control levels obtained from the contralateral RVLM under the same experimental conditions.
Drugs
Abn CBD and O-1918 (1,3-dimethoxy-5-methyl-2-[(1R,6R)-3-methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]benzene) were purchased from Cayman Chemical (Ann Arbor, MI). Methyl acetate, wortmannin, PD98059, forskolin, and DMSO were purchased from Sigma-Aldrich (St. Louis, MO). L-NIO and NPLA were purchased from Tocris Biosciences (Ellisville, MO) and dissolved in sterile saline. Sterile saline was purchased from B. Braun Medical (Irvine, CA). DMSO was used as the vehicle for PD98059, wortmannin, and forskolin. Methyl acetate was used as the vehicle for Abn CBD, O-1918, and NAGly. Methyl acetate and DMSO, tested in at least three animals, did not cause any significant changes in mean arterial pressure (MAP) and HR from the basal levels. All intra-RVLM injections were of equal volume (80 nl).
Data and Statistical Analyses
All values are expressed as the mean ± S.E.M change from their respective baselines. All other statistical analyses were performed using a one-way or repeated-measures analysis of variance with the Bonferroni post hoc test and t test. Prism 5.0 software (GraphPad Software, Inc., San Diego, CA) was used to perform statistical analysis. P < 0.05 was considered significant.
Results
Inhibition of RVLM PI3K/Akt, ERK1/2, or nNOS or Elevation in RVLM cAMP Levels Attenuated Abn CBD–Evoked Hypotensive Response.
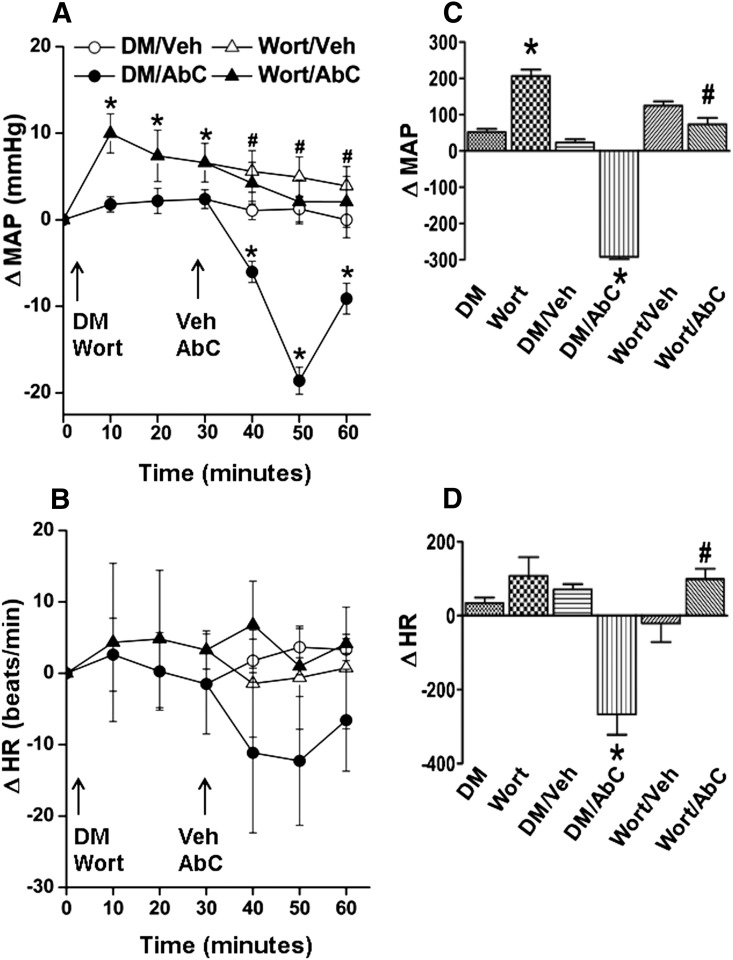
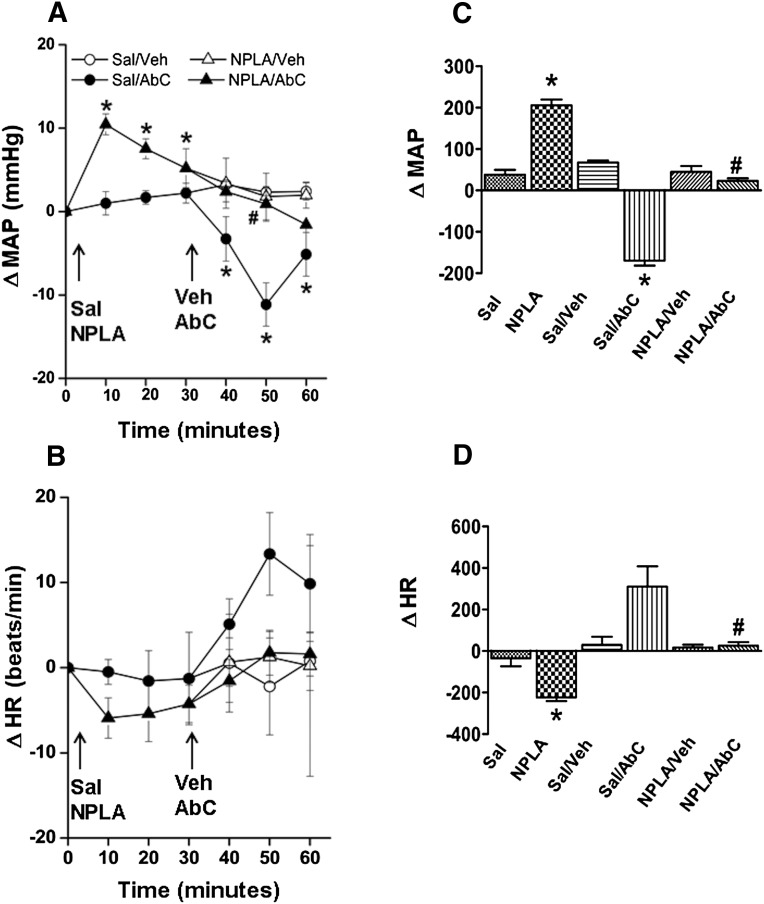
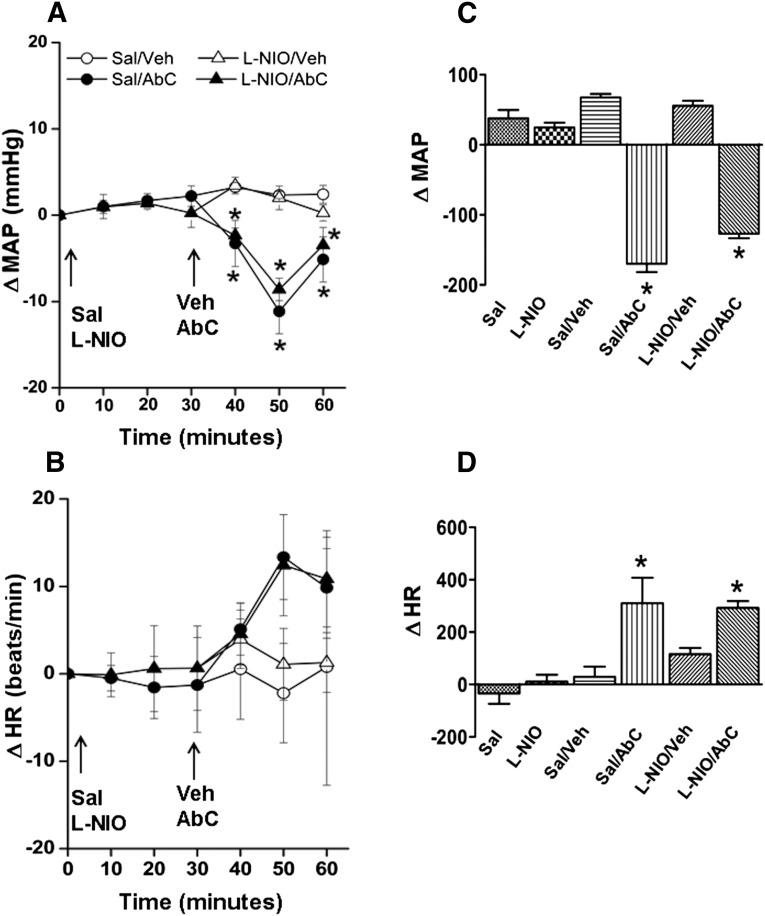
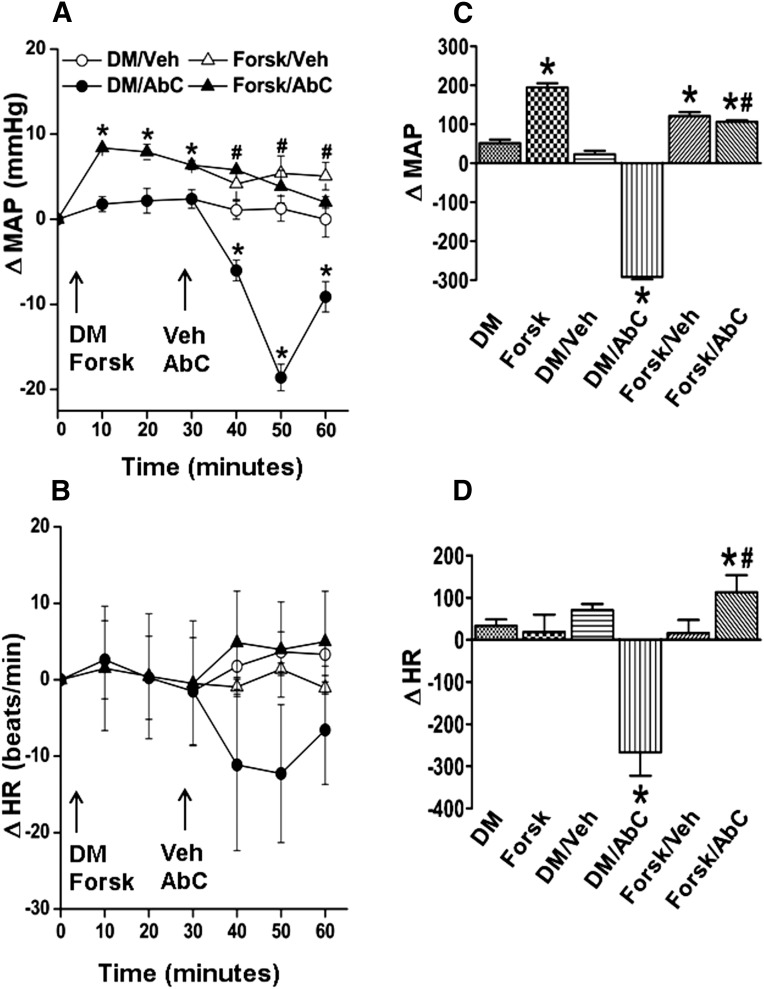
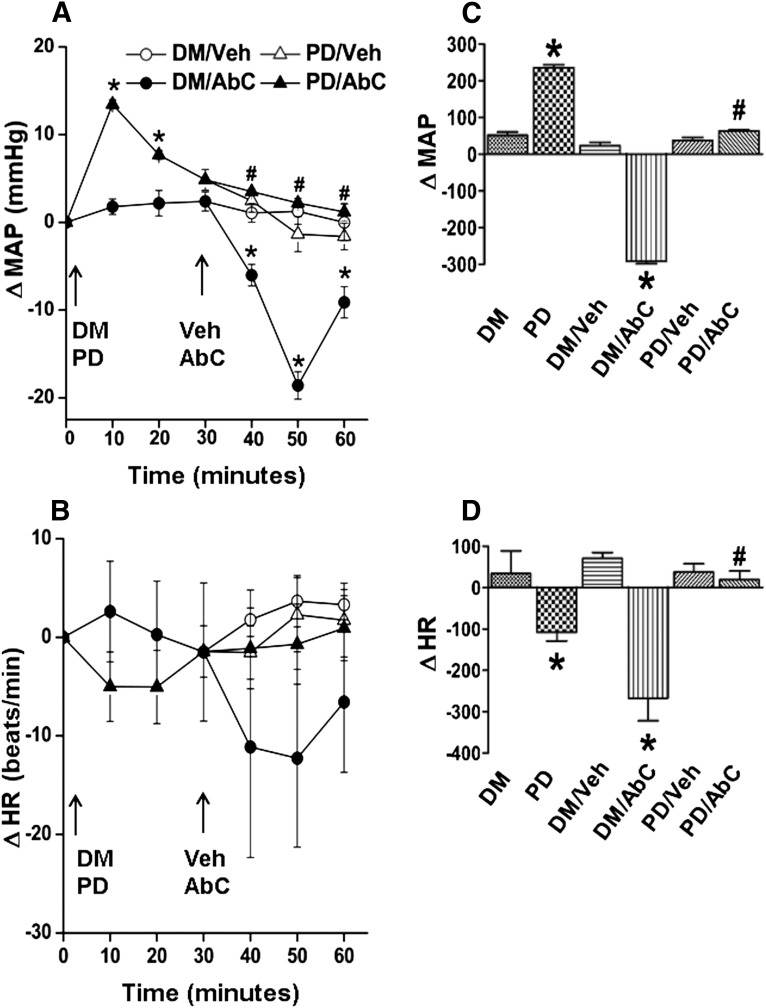
MAP (in millimeters of mercury) and HR (in beats per minute) after pretreatment time (30 minutes) and before Abn CBD or its vehicle administration were similar (Table 1). Compared with the vehicle (DMSO, methyl acetate, or saline), inhibition of RVLM PI3K/Akt (wortmannin; 100 nmol), ERK1/2 (PD98059; 50 μmol), or nNOS (NPLA; 250 pmol) caused significant (P < 0.05) BP elevation, which subsided to within control levels before intra-RVLM Abn CBD or vehicle administration (Figs. 1–4). Each of these pharmacological inhibitors significantly (P < 0.05) attenuated Abn CBD (0.4 μg)–evoked hypotension (Figs. 1–4). On the other hand, RVLM eNOS (L-NIO; 100 pmol) inhibition had no effect on BP or on the Abn CBD-evoked hypotension (Fig. 3, A and C). The changes in HR in the different treatment groups were not significantly different. However, when the HR responses were expressed as the area under the curve, a significant bradycardic response occurred when Abn CBD was administered after DMSO, but not after saline (Figs. 1B–4B). Intra-RVLM activation of AC (elevation of cAMP levels) by forskolin (50 μmol) significantly (P < 0.05) increased BP (Fig. 5, A and C), but had no effect on HR (Fig. 5, B and D). Furthermore, forskolin pretreatment abrogated the central GPR18-mediated hypotensive (Fig. 5, A and C) and bradycardic (Fig. 5D) responses.
TABLE 1.
Values of MAP and HR at the end of pretreatment (30 minutes) and immediately before treatment with the indicated intervention or its vehicle
Values are the mean ± S.E.M.
| Pretreatment/Treatment | Rats per Group | MAP | HR |
|---|---|---|---|
| n | mm Hg | beats/min | |
| DMSO/vehicle | 3 | 121 ± 3 | 359 ± 10 |
| Saline/vehicle | 3 | 116 ± 5 | 372 ± 8 |
| DMSO/Abn CBD | 3 | 111 ± 8 | 363 ± 7 |
| Saline/Abn CBD | 3 | 113 ± 7 | 330 ± 18 |
| PD98059/vehicle | 6 | 115 ± 3 | 324 ± 11 |
| PD98059/Abn CBD | 6 | 120 ± 1 | 367 ± 12 |
| Wortmannin/vehicle | 6 | 118 ± 5 | 328 ± 14 |
| Wortmannin/Abn CBD | 6 | 117 ± 6 | 349 ± 16 |
| NPLA/vehicle | 6 | 123 ± 5 | 342 ± 9 |
| NPLA/Abn CBD | 6 | 114 ± 5 | 361 ± 11 |
| L-NIO/vehicle | 6 | 122 ± 9 | 335 ± 10 |
| L-NIO/Abn CBD | 6 | 119 ± 2 | 357 ± 13 |
| Forskolin/vehicle | 6 | 112 ± 7 | 345 ± 17 |
| Forskolin/Abn CBD | 6 | 109 ± 8 | 376 ± 15 |
Fig. 1.
(A and B) Time course changes in MAP (A) and HR (B) after intra-RVLM Abn CBD (0.4 μg) or an equal volume of vehicle in conscious rats pretreated (30 minutes earlier) with the PI3K/Akt inhibitor wortmannin (100 nmol) or equal volume of its vehicle (DMSO; diluted 1:16 in ACSF). (C and D) AUC values generated from the time course data show that inhibition of Akt phosphorylation (wortmannin) abrogates the GPR18 (Abn CBD)-mediated hypotensive response. The responses from the two groups that received the same pretreatment (DMSO or wortmannin) were combined for clarity. The left two bars in the AUC graph show pretreatment. Six rats per group were used except for the DMSO plus vehicle or Abn CBD groups (n = 3 each). *P < 0.05 versus control (vehicle); #P < 0.05 versus Abn CBD. AbC, Abn CBD; ACSF, artificial cerebrospinal fluid; AUC, area under the curve; ΔHR, change in HR; ΔMAP, change in MAP; DM, DMSO; Veh, vehicle (methyl acetate); Wort, wortmannin.
Fig. 4.
(A and B) Changes in MAP (A) and HR (B) after intra-RVLM microinjections of Abn CBD (0.4 μg) or an equal volume of vehicle in conscious male Sprague-Dawley rats pretreated (30 minutes earlier) with NPLA (selective nNOS inhibitor; 250 pmol). Pretreatment with NPLA virtually abolished the GPR18 (Abn CBD)-mediated hypotensive response, which suggests nNOS involvement in GPR18 signaling. (C and D) AUC data generated from the time course values over the pretreatment (0–30 minutes) and treatment (30–60 minutes) periods. The left two bars in the AUC graph show pretreatment. All pretreatment data from groups of rats that received the same drugs were combined for clarity. Six rats per group were used except for the saline plus vehicle or Abn CBD groups (n = 3 each). *P < 0.05 versus control (vehicle); #P < 0.05 versus Abn CBD. AbC, Abn CBD; AUC, area under the curve; ΔHR, change in HR; ΔMAP, change in MAP; Sal, saline; Veh, vehicle (methyl acetate).
Fig. 3.
(A and B) Changes in MAP (A) and HR (B) after intra-RVLM microinjections of Abn CBD (0.4 μg) or equal volume of vehicle in conscious male Sprague-Dawley rats pretreated (30 minutes earlier) with L-NIO (selective eNOS inhibitor; 100 pmol). Pretreatment with L-NIO did not affect the GPR18 (Abn CBD)-mediated hypotensive response, which rules out eNOS involvement in GPR18 signaling. (C and D) AUC data generated from the time course values over the pretreatment (0–30 minutes) and treatment (30–60 minute) periods. The left two bars in the AUC graph show pretreatment. All pretreatment data from groups of rats that received the same drug were combined for clarity. Six rats per group were used except for the saline plus vehicle or Abn CBD groups (n = 3 each). *P < 0.05 versus control (vehicle); AbC, Abn CBD; AUC, area under the curve; ΔHR, change in HR; ΔMAP, change in MAP; Sal, saline; Veh, vehicle (methyl acetate).
Fig. 5.
(A and B) Changes in MAP (A) and HR (B) after intra-RVLM microinjections of Abn CBD (0.4 μg) or an equal volume of vehicle in conscious male Sprague-Dawley rats pretreated (30 minutes earlier) with forskolin (cAMP elevation; 50 μmol). (C and D) AUC data generated from the time course values over the pretreatment (0–30 minutes) and treatment (30–60 minutes) periods. The left two bars in the AUC graph show pretreatment. All pretreatment data from groups of rats that received the same drugs were combined for clarity. Six rats per group were used except for the DMSO plus vehicle or Abn CBD groups (n = 3 each). *P < 0.05 versus control (vehicle); #P < 0.05 versus Abn CBD. AbC, Abn CBD; AUC, area under the curve; ΔHR, change in HR; ΔMAP, change in MAP; Forsk, forskolin; Veh, vehicle (methyl acetate).
Fig. 2.
(A and B) Changes in MAP (A) and HR (B) after intra-RVLM microinjections of either DMSO (diluted 1:16 in ACSF) or PD98059 (50 μmol) at 0 minutes and subsequent vehicle (methyl acetate) or Abn CBD (0.4 μg) microinjection. Pretreatment with PD98059 abrogated the GPR18 (Abn CBD)-mediated hypotensive response. (C and D) AUC data generated from the time course values over the pretreatment (0–30 minutes) and treatment (30–60 minutes) periods. The left two bars in the AUC graph show pretreatment. Compared with vehicle, PD98059 caused significant elevation in BP (A and C). Abn CBD caused significant reduction in BP, and this response was abrogated in PD98059 pretreated rats (A and C). All pretreatment data obtained from groups of rats that received the same drug were combined for clarity. Six rats per group were used except DMSO plus vehicle or Abn CBD groups (n = 3 each). *P < 0.05 versus control (vehicle); #P < 0.05 versus Abn CBD. AbC, Abn CBD; ACSF, artificial cerebrospinal fluid; AUC, area under the curve; ΔHR, change in HR; ΔMAP, change in MAP; DM, DMSO; PD, PD98059; Veh, vehicle (methyl acetate).
Akt, ERK1/2, or nNOS Inhibition or cAMP Elevation Abrogates GPR18-Mediated Molecular Events in the RVLM.
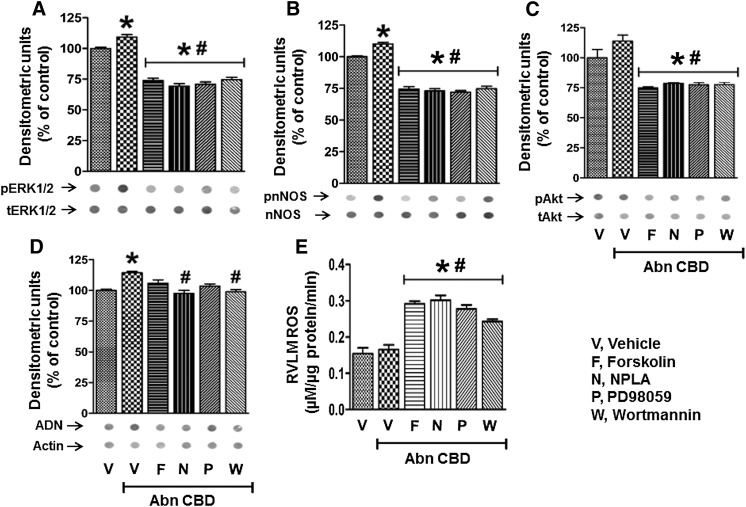
Dot blot analyses were used to permit multiple measurements of the targeted proteins as well as ADN and ROS levels in the limited amount of RVLM tissues collected from treatment and control groups. Importantly, this methodology was validated by the similarity of the Abn CBD–evoked increases in Akt, ERK1/2, and nNOS phosphorylation measured by dot blot (Fig. 6) and Western blot analyses in a preliminary study (Supplemental Fig. 1). Intra-RVLM pretreatment with wortmannin, NPLA, PD98059, or forskolin significantly (P < 0.05) attenuated the GPR18 (Abn CBD)-mediated increases in phosphorylation of ERK1/2, Akt, and nNOS, and in ADN levels in the RVLM (Fig. 6, A–D). Furthermore, pharmacologic inhibition of Akt, ERK1/2, or nNOS phosphorylation or elevation of cAMP abrogated GPR18-mediated hypotension (Figs. 1–5), and resulted in significant elevation in ROS levels in the RVLM (Fig. 6E). These neurochemical findings paralleled the BP responses (Figs. 1–5).
Fig. 6.
(A to D) Dot blots showing the effects of Abn CBD alone or after pretreatment with forskolin, NPLA, PD98059, or wortmannin on pERK1/2 (A), pAkt (B), p-nNOS (C), and ADN (D) levels in the RVLM. Data are presented as the integrated density ratio of the phosphorylated protein to its total protein and are expressed as the percentage of control (vehicle). (E) RVLM ROS levels after treatment with Abn CBD in the absence or presence of forskolin, NPLA, PD98059, or wortmannin. Values are the mean ± S.E.M. of 3–6 observations. *P < 0.05 versus vehicle; #P < 0.05 versus Abn CBD values. F, forskolin; N, NPLA; P, PD98059; V, vehicle; W, wortmannin.
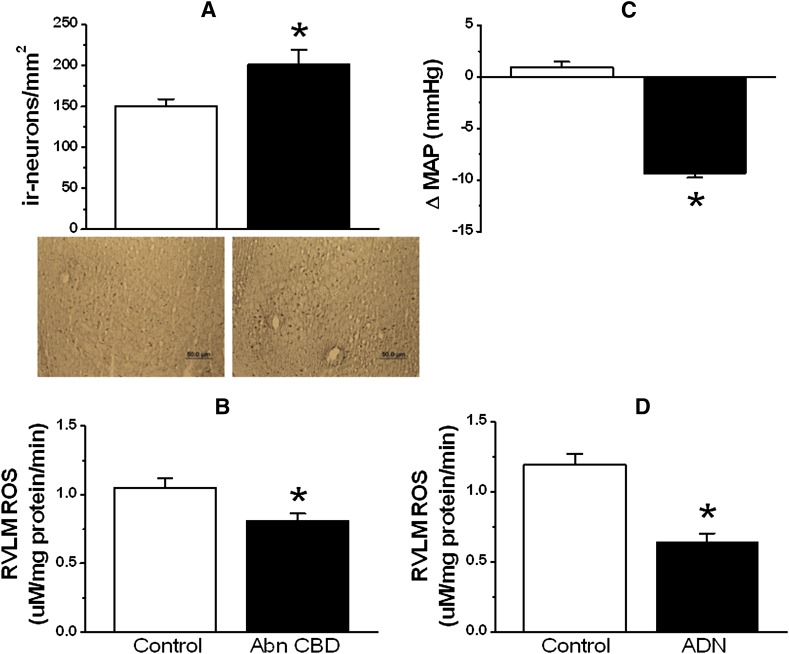
Finally, in two additional groups of rats, we determined whether the time of measurement of RVLM ADN levels (after Abn CBD administration) influences the outcome and whether microinjection of a low dose of ADN reduces BP and RVLM ROS levels. Notably, measurements in tissues collected at 30 minutes after intra-RVLM Abn CBD, when the hypotensive response was dissipating (Fig. 1), revealed a minute (15%) elevation in ADN (Fig. 6D) and no change in ROS (Fig. 6E) in the RVLM. However, when immunohistochemical measurements were conducted in tissues of rats (n = 5) euthanized 15 minutes after intra-RVLM Abn CBD (during the maximal fall in BP), the RVLM exhibited significantly (P < 0.05) greater ADN-immunoreactive neurons (Fig. 7A) and lower ROS (Fig. 7B) levels, compared with the corresponding control levels in the contralateral RVLM. Furthermore, microinjection of a relatively low dose (0.5 pmol) of ADN in another group of rats (n = 4) caused significant (P < 0.05) reductions in BP (Fig. 7C) and RVLM ROS (Fig. 7D) levels. Baseline MAP and HR values in these two groups were not significantly different from the values of the other groups shown in Table 1 (data not shown).
Fig. 7.
(A and B) Left panels show changes in the number of ADN-immunoreactive neurons along with representative immunohistochemical images (A) and RVLM ROS levels observed in the RVLM of rats (n = 5) euthanized 15 minutes after Abn CBD (0.4 μg) microinjection (B). (C and D) Right panels show changes in MAP (C) and RVLM ROS (D) measured in brains (n = 4) collected during the hypotensive response elicited by intra-RVLM ADN (0.5 pmol) microinjection. *P < 0.05 versus control values (vehicle in case of MAP and the corresponding ADN-immunoreactive neurons and ROS levels in the contralateral RVLM). ΔMAP, change in MAP; ir, immunoreactive. Bar, 50 μm.
Discussion
We tested the hypothesis that GPR18-mediated phosphorylation of RVLM Akt and ERK1/2, triggered by cAMP inhibition, underlies the nNOS (NO)-dependent elevation in ADN and the subsequent reductions in RVLM ROS and BP. The most important findings of this study are as follows. First, activation of RVLM GPR18 (Abn CBD) increased Akt, ERK1/2, and nNOS phosphorylation, as well as ADN levels in the RVLM. Second, pharmacological inhibition of RVLM PI3K/Akt, ERK1/2, or nNOS or elevation of cAMP increased the RVLM ROS level and attenuated the GPR18-mediated increases in the phosphorylation of Akt, ERK1/2, and nNOS, as well as ADN and hypotension. Together, these findings suggest a pivotal role for Akt-ERK1/2-nNOS activation and cAMP reduction in the favorable ADN-dependent redox and BP responses mediated by RVLM GPR18 in conscious rats.
Our findings (Penumarti and Abdel-Rahman, 2014) inferred a role for RVLM ADN in GPR18-mediated hypotension because GPR18 activation increases local ADN and NO and lowers BP, and intra-RVLM ADN reduces RVLM ROS and BP. Building on these findings, this study contributed new knowledge on the molecular mechanisms of central GPR18-mediated hypotension. We investigated the role of NO because the balance between mediators and suppressors of ROS in the RVLM controls BP (Fridovich, 1978; Kishi et al., 2004; Chan et al., 2006). Notably, the NOS isoform(s) implicated in the GPR18-mediated increase in RVLM NO and its potential link to ADN elevation, which reduces oxidative stress (Song et al., 2013), are not known.
Although GPR18 activation enhances Akt and ERK1/2 in vitro (McHugh et al., 2010), the effect of GPR18 activation on mitogen-activated protein kinase or NOS phosphorylation in any brain structure is not known. We hypothesized that phosphorylation of nNOS, and its interrelated kinases (Akt and ERK1/2) in the RVLM, plays a major role in GPR18-mediated NO and ADN elevation as well as the subsequent hypotension. Here, we show that Abn CBD enhanced phosphorylation of Akt, ERK1/2, and nNOS in the RVLM. These neurochemical responses seem to be GPR18 mediated because local GPR18 blockade (O-1918) produced opposite effects and abrogated the Abn CBD–evoked responses (Supplemental Fig. 1). We focused on the constitutive NOS isoforms because the rapidly developing GPR18-mediated hypotension (Fig. 3) precluded potential inducible NOS contribution to GPR18-mediated hypotension. Notably, nNOS is the major NOS isoform in the brainstem (Chan et al., 2001). Nonetheless, although our preliminary findings agree with similar increases in Akt and ERK1/2 phosphorylation in vitro (McHugh et al., 2010, 2012), it was important to determine whether these neurochemical responses are causally linked to GPR18-mediated ADN elevation and hypotension.
We conducted pharmacological and ex vivo studies to elucidate the role of Akt, ERK1/2, and eNOS phosphorylation in the GPR18-mediated increases in NO and ADN, as well as hypotension. We also investigated a possible role for AC inhibition (reduced cAMP) in these neurochemical and BP responses because activation of GPR18, a Gi/o-coupled receptor, reduces cAMP in vitro (McHugh et al., 2010) and activation of the α2-adrenergic receptor, another Gi/o-coupled receptor, enhances brainstem ERK1/2 and nNOS phosphorylation and lowers BP (Zhang and Abdel-Rahman, 2005; Nassar and Abdel-Rahman, 2008). Because cAMP measurement in the scarce RVLM tissue was not feasible, we tested our hypothesis by microinjecting forskolin to generate cAMP (Edwards and Paton, 1999). We show that intra-RVLM inhibition of PI3K/Akt (wortmannin) or ERK1/2 (PD98059) or cAMP elevation (forskolin) virtually abolished the hypotension (Figs. 1–5) and the enhanced phosphorylation of Akt, ERK1/2, and nNOS, as well as ADN levels in the RVLM (Fig. 6) caused by Abn CBD. Furthermore, selective nNOS (NPLA), but not eNOS (L-NIO), inhibition attenuated GPR18-mediated hypotension (Figs. 3 and 4) as well as nNOS phosphorylation and elevation of ADN levels in the RVLM (Fig. 6D). Importantly, these pharmacological interventions resulted in higher ROS levels in the RVLM (Fig. 6E), which supports a possible role for tonic activation of GPR18 and the downstream signaling pathways in central BP regulation. This conclusion gains credence from the elevations of BP and RVLM ROS (Penumarti and Abdel-Rahman, 2014), and RVLM pERK1/2, p-nNOS, and pAkt levels (Supplemental Fig. 1) caused by intra-RVLM GPR18 blockade (O-1918). Collectively, these findings support a causal role for nNOS phosphorylation, at least partly, via the PI3K-Akt-ERK1/2 activation and cAMP reduction in GPR18-mediated hypotension.
The role of Akt in GPR18-mediated hypotension may offer a plausible explanation for the functional antagonism between GPR18 and CB1R in the RVLM (Penumarti and Abdel-Rahman, 2014). Although GPR18 activation enhanced Akt phosphorylation in the RVLM in this study, our previous study showed that CB1R suppressed Akt phosphorylation in the RVLM (Ibrahim and Abdel-Rahman, 2012b). These contrasting responses are functionally relevant and might be linked to GABA/Akt interaction in the RVLM because of the following reasons. First, inhibition of Akt phosphorylation (wortmannin) attenuated GPR18-mediated hypotension (Fig. 1) and exacerbated CB1R-mediated hypertension (Ibrahim and Abdel-Rahman, 2012b). Second, GABA enhances Akt phosphorylation (Xu et al., 2008) and lowers BP (Peng et al., 2011; Wu et al., 2012). Third, suppressed Akt phosphorylation and GABA activity in the RVLM contribute to CB1R-dependent hypertension (Ibrahim and Abdel-Rahman, 2011). Fourth, RVLM GPR18 blockade (O-1918), which increased BP (Penumarti and Abdel-Rahman, 2014), inhibited Akt phosphorylation in the RVLM (Supplemental Fig. 1). More studies are warranted to determine whether GPR18-dependent Akt phosphorylation enhances RVLM GABA signaling. Nonetheless, these findings support a role for Akt phosphorylation in central GPR18 signaling. Furthermore, the current pharmacological and signal transduction findings support the concept of “biased agonism” suggested by Console-Bram et al. (2014) as a reasonable explanation for reported findings that questioned GPR18 as being the Abn CBD receptor (Yin et al., 2009; Lu et al., 2013).
A critical role for ADN in RVLM GPR18 signaling and the subsequent hypotensive response must be cautiously accepted because of two limitations of this study. First, we did not investigate the effect of ADN receptor blockade on the GPR18-mediated hypotension. Notably, research on the role of ADN in neural central BP control is in its infancy, and there are no available ADN receptor blockers. Nonetheless, our findings identified important molecular mechanisms for the GPR18-mediated elevation in ADN in the RVLM (Figs. 6 and 8). Second, Abn CBD produced a minute increase in ADN (Fig. 6D), and did not reduce the RVLM ROS level (Fig. 6E), which raised a concern about ADN involvement in GPR18 signaling. Although ADN receptors are widely distributed in the brain (Thundyil et al., 2012), there is a debate on whether ADN is generated within the brain or transported from circulation to activate its receptors. Activation of the latter in the nucleus tractus solitarius by ADN (1 pmol), which was considered comparable to its level in that area, lowered BP (Hoyda et al., 2009). Building on the findings of that group, we showed that intra-RVLM ADN (0.25–4 pmol) dose dependently reduced BP and RVLM ROS, but we only measured ROS after the 4-pmol dose (Penumarti and Abdel-Rahman, 2014). Here, we show that a low dose (0.5 pmol) of intra-RVLM ADN reduced RVLM ROS and BP (Fig. 7), which supports the possible contribution of ADN to Abn CBD–evoked reduction in BP. Furthermore, we reasoned that measurement of ADN in the whole RVLM tissue, and particularly at a time when the hypotensive response was dissipating (30 minutes after Abn CBD), might account for the minute elevation in ADN and the lack of reduction in ROS in the RVLM of Abn CBD–treated rats (Fig. 6, D and E). This notion was supported by a much higher elevation in ADN and a significant reduction in ROS level in RVLM tissues collected 15 minutes after intra-RVLM Abn CBD, during the maximal hypotensive response (Fig. 7).
Fig. 8.
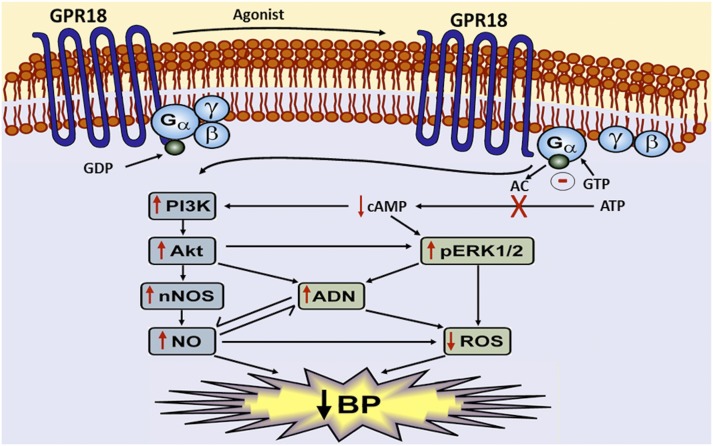
Schematic presentation of the neurochemical (RVLM) and BP responses elicited by activating central GPR18. Activation of intra-RVLM GPR18 inhibits cAMP leading to enhanced pERK1/2 and pAkt production, which causes p-nNOS (NO)–dependent elevation in ADN, and ultimately reductions in RVLM ROS and BP.
It is also important to comment on the interaction between ADN and NO and their link to ROS in cannabinoid receptor signaling. First, our findings support a role for NO in ADN-evoked dilation of retinal blood vessels (Lin et al., 2013) although NO was derived via nNOS (this study) and eNOS (reported study). It is likely that ADN interaction with different NOS isoforms depends on the tissue type. Second, our new findings with ADN, which extended our recent findings (Penumarti and Abdel-Rahman, 2014), highlight a functional role for ADN in cannabinoid receptor regulation of oxidative stress, and perhaps in the opposite BP responses mediated by RVLM GPR18 and CB1R because of the following. First, GPR18 enhances (our studies) ADN levels, whereas CB1R suppresses ADN levels, and ADN responses parallel changes in the ROS level (Nanayakkara et al., 2012; Song et al., 2013). Second, RVLM GPR18 mediates hypotension (this study), whereas CB1R mediates elevation in BP (Ibrahim and Abdel-Rahman, 2011) and masks GPR18-mediated hypotension (Penumarti and Abdel-Rahman, 2014). Together, these findings implicate RVLM nNOS- and Akt-dependent elevation in ADN levels in the GPR18-mediated reductions in RVLM ROS and BP.
In conclusion, this study yields new insight into the RVLM signaling pathways implicated in GPR18-mediated hypotension. We provide the first evidence that activation of the RVLM PI3K-Akt-ERK1/2-nNOS-ADN network, triggered at least partly by a local reduction in the cAMP level, plays a pivotal role in RVLM GPR18-mediated hypotension (Fig. 8). The present comprehensive in vivo and ex vivo evidence of a favorable role for RVLM GPR18 in BP control highlights this putative cannabinoid receptor as a potential novel target for new antihypertensive drugs. Future studies are warranted to delineate the role of GPR18 in hypertension neurobiology.
Supplementary Material
Acknowledgments
The authors thank Kui Sun for technical assistance.
Abbreviations
- Abn CBD
abnormal cannabidiol (trans-4-[3-methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]-5-pentyl-1,3-benzenediol)
- AC
adenylyl cyclase
- ADN
adiponectin
- BP
blood pressure
- CB1R
cannabinoid receptor 1
- DMSO
dimethylsulfoxide
- ERK1/2
extracellular signal-regulated kinase 1/2
- eNOS
endothelial nitric oxide synthase
- GPR18
G protein–coupled receptor 18
- HR
heart rate
- L-NIO
N5-(1-iminoethyl)-l-ornithine
- MAP
mean arterial pressure
- NAGly
N-arachidonoyl glycine
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
- NOS
nitric oxide synthase
- NPLA
Nω-propyl-l-arginine hydrochloride
- O-1918
1,3-dimethoxy-5-methyl-2-[(1R,6R)-3-methyl-6-(1-methylethenyl)-2-cyclohexen-1-yl]benzene
- PD98059
2-(2-amino-3-methoxyphenyl)-4H-1-benzopyran-4-one
- PI3K
phosphoinositide 3-kinase
- ROS
reactive oxygen species
- RVLM
rostral ventrolateral medulla
Authorship Contributions
Participated in research design: Penumarti, Abdel-Rahman.
Conducted experiments: Penumarti.
Performed data analysis: Penumarti.
Wrote or contributed to the writing of the manuscript: Penumarti, Abdel-Rahman.
Footnotes
This work was supported in part by the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grant 2R01-AA07839-19].
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. (1995) PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem 270:27489–27494 [DOI] [PubMed] [Google Scholar]
- Alexander SP. (2012) So what do we call GPR18 now? Br J Pharmacol 165:2411–2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SH, Chan JY. (2014) Brain stem NOS and ROS in neural mechanisms of hypertension. Antioxid Redox Signal 20:146–163 [DOI] [PubMed] [Google Scholar]
- Chan SH, Tai MH, Li CY, Chan JY. (2006) Reduction in molecular synthesis or enzyme activity of superoxide dismutases and catalase contributes to oxidative stress and neurogenic hypertension in spontaneously hypertensive rats. Free Radic Biol Med 40:2028–2039 [DOI] [PubMed] [Google Scholar]
- Chan SH, Wang LL, Tseng HL, Chan JY. (2007) Upregulation of AT1 receptor gene on activation of protein kinase Cbeta/nicotinamide adenine dinucleotide diphosphate oxidase/ERK1/2/c-fos signaling cascade mediates long-term pressor effect of angiotensin II in rostral ventrolateral medulla. J Hypertens 25:1845–1861 [DOI] [PubMed] [Google Scholar]
- Chan SH, Wang LL, Wang SH, Chan JY. (2001) Differential cardiovascular responses to blockade of nNOS or iNOS in rostral ventrolateral medulla of the rat. Br J Pharmacol 133:606–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Console-Bram L, Brailoiu E, Brailoiu GC, Sharir H, Abood ME. (2014) Activation of GPR18 by cannabinoid compounds: a tale of biased agonism. Br J Pharmacol 171:3908–3917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards E, Paton JF. (1999) 5-HT(4) receptors in nucleus tractus solitarii attenuate cardiopulmonary reflex in anesthetized rats. Am J Physiol 277:H1914–H1923 [DOI] [PubMed] [Google Scholar]
- El-Mas MM, Fan M, Abdel-Rahman AA. (2009) Facilitation of myocardial PI3K/Akt/nNOS signaling contributes to ethanol-evoked hypotension in female rats. Alcohol Clin Exp Res 33:1158–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. (1978) The biology of oxygen radicals. Science 201:875–880 [DOI] [PubMed] [Google Scholar]
- Guyenet PG. (2006) The sympathetic control of blood pressure. Nat Rev Neurosci 7:335–346 [DOI] [PubMed] [Google Scholar]
- Hirooka Y. (2008) Role of reactive oxygen species in brainstem in neural mechanisms of hypertension. Auton Neurosci 142:20–24 [DOI] [PubMed] [Google Scholar]
- Hoyda TD, Smith PM, Ferguson AV. (2009) Adiponectin acts in the nucleus of the solitary tract to decrease blood pressure by modulating the excitability of neuropeptide Y neurons. Brain Res 1256:76–84 [DOI] [PubMed] [Google Scholar]
- Ibrahim BM, Abdel-Rahman AA. (2011) Role of brainstem GABAergic signaling in central cannabinoid receptor evoked sympathoexcitation and pressor responses in conscious rats. Brain Res 1414:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim BM, Abdel-Rahman AA. (2012a) Differential modulation of brainstem phosphatidylinositol 3-kinase/Akt and extracellular signal-regulated kinase 1/2 signaling underlies WIN55,212-2 centrally mediated pressor response in conscious rats. J Pharmacol Exp Ther 340:11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim BM, Abdel-Rahman AA. (2012b) Enhancement of rostral ventrolateral medulla neuronal nitric-oxide synthase-nitric-oxide signaling mediates the central cannabinoid receptor 1-evoked pressor response in conscious rats. J Pharmacol Exp Ther 341:579–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute for Laboratory Animal Research (2011) Guide for the Care and Use of Laboratory Animals, 8th ed, National Research Council, Washington, DC [Google Scholar]
- Kishi T, Hirooka Y, Kimura Y, Ito K, Shimokawa H, Takeshita A. (2004) Increased reactive oxygen species in rostral ventrolateral medulla contribute to neural mechanisms of hypertension in stroke-prone spontaneously hypertensive rats. Circulation 109:2357–2362 [DOI] [PubMed] [Google Scholar]
- Kohno M, Hasegawa H, Inoue A, Muraoka M, Miyazaki T, Oka K, Yasukawa M. (2006) Identification of N-arachidonylglycine as the endogenous ligand for orphan G-protein-coupled receptor GPR18. Biochem Biophys Res Commun 347:827–832 [DOI] [PubMed] [Google Scholar]
- Lin T, Qiu Y, Liu Y, Mohan R, Li Q, Lei B. (2013) Expression of adiponectin and its receptors in type 1 diabetes mellitus in human and mouse retinas. Mol Vis 19:1769–1778 [PMC free article] [PubMed] [Google Scholar]
- Lu VB, Puhl HL, 3rd, Ikeda SR. (2013) N-Arachidonyl glycine does not activate G protein-coupled receptor 18 signaling via canonical pathways. Mol Pharmacol 83:267–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollum L, Howlett AC, Mukhopadhyay S. (2007) Anandamide-mediated CB1/CB2 cannabinoid receptor—independent nitric oxide production in rabbit aortic endothelial cells. J Pharmacol Exp Ther 321:930–937 [DOI] [PubMed] [Google Scholar]
- McHugh D, Hu SS, Rimmerman N, Juknat A, Vogel Z, Walker JM, Bradshaw HB. (2010) N-arachidonoyl glycine, an abundant endogenous lipid, potently drives directed cellular migration through GPR18, the putative abnormal cannabidiol receptor. BMC Neurosci 11:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh D, Page J, Dunn E, Bradshaw HB. (2012) Δ(9) -Tetrahydrocannabinol and N-arachidonyl glycine are full agonists at GPR18 receptors and induce migration in human endometrial HEC-1B cells. Br J Pharmacol 165:2414–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanayakkara G, Kariharan T, Wang L, Zhong J, Amin R. (2012) The cardio-protective signaling and mechanisms of adiponectin. Am J Cardiovas Dis 2:253–266 [PMC free article] [PubMed] [Google Scholar]
- Nassar N, Abdel-Rahman AA. (2008) Brainstem phosphorylated extracellular signal-regulated kinase 1/2-nitric-oxide synthase signaling mediates the adenosine A2A-dependent hypotensive action of clonidine in conscious aortic barodenervated rats. J Pharmacol Exp Ther 324:79–85 [DOI] [PubMed] [Google Scholar]
- Nassar NN, Li G, Strat AL, Abdel-Rahman AA. (2011) Enhanced hemeoxygenase activity in the rostral ventrolateral medulla mediates exaggerated hemin-evoked hypotension in the spontaneously hypertensive rat. J Pharmacol Exp Ther 339:267–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offertáler L, Mo FM, Bátkai S, Liu J, Begg M, Razdan RK, Martin BR, Bukoski RD, Kunos G. (2003) Selective ligands and cellular effectors of a G protein-coupled endothelial cannabinoid receptor. Mol Pharmacol 63:699–705 [DOI] [PubMed] [Google Scholar]
- Peng JF, Wu ZT, Wang YK, Yuan WJ, Sun T, Ni X, Su DF, Wang W, Xu MJ, Wang WZ. (2011) GABAergic mechanism in the rostral ventrolateral medulla contributes to the hypotension of moxonidine. Cardiovasc Res 89:473–481 [DOI] [PubMed] [Google Scholar]
- Penumarti A, Abdel-Rahman AA. (2014) The novel endocannabinoid receptor GPR18 is expressed in the rostral ventrolateral medulla and exerts tonic restraining influence on blood pressure. J Pharmacol Exp Ther 349:29–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyedabadi M, Goodchild AK, Pilowsky PM. (2001) Differential role of kinases in brain stem of hypertensive and normotensive rats. Hypertension 38:1087–1092 [DOI] [PubMed] [Google Scholar]
- Song W, Huo T, Guo F, Wang H, Wei H, Yang Q, Dong H, Wang Q, Xiong L. (2013) Globular adiponectin elicits neuroprotection by inhibiting NADPH oxidase-mediated oxidative damage in ischemic stroke. Neuroscience 248C:136–144 [DOI] [PubMed] [Google Scholar]
- Sved AF, Ito S, Sved JC. (2003) Brainstem mechanisms of hypertension: role of the rostral ventrolateral medulla. Curr Hypertens Rep 5:262–268 [DOI] [PubMed] [Google Scholar]
- Thundyil J, Pavlovski D, Sobey CG, Arumugam TV. (2012) Adiponectin receptor signalling in the brain. Br J Pharmacol 165:313–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda K, Tsuda S, Nishio I. (2003) Role of alpha2-adrenergic receptors and cyclic adenosine monophosphate-dependent protein kinase in the regulation of norepinephrine release in the central nervous system of spontaneously hypertensive rats. J Cardiovasc Pharmacol 42 (Suppl 1):S81–S85 [DOI] [PubMed] [Google Scholar]
- Wu KL, Chen CH, Shih CD. (2012) Nontranscriptional activation of PI3K/Akt signaling mediates hypotensive effect following activation of estrogen receptor β in the rostral ventrolateral medulla of rats. J Biomed Sci 19:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Li C, Yin XH, Zhang GY. (2008) Additive neuroprotection of GABA A and GABA B receptor agonists in cerebral ischemic injury via PI-3K/Akt pathway inhibiting the ASK1-JNK cascade. Neuropharmacology 54:1029–1040 [DOI] [PubMed] [Google Scholar]
- Yin H, Chu A, Li W, Wang B, Shelton F, Otero F, Nguyen DG, Caldwell JS, Chen YA. (2009) Lipid G protein-coupled receptor ligand identification using beta-arrestin PathHunter assay. J Biol Chem 284:12328–12338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Abdel-Rahman AA. (2002) The hypotensive action of rilmenidine is dependent on functional N-methyl-D-aspartate receptor in the rostral ventrolateral medulla of conscious spontaneously hypertensive rats. J Pharmacol Exp Ther 303:204–210 [DOI] [PubMed] [Google Scholar]
- Zhang J, Abdel-Rahman AA. (2005) Mitogen-activated protein kinase phosphorylation in the rostral ventrolateral medulla plays a key role in imidazoline (i1)-receptor-mediated hypotension. J Pharmacol Exp Ther 314:945–952 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.