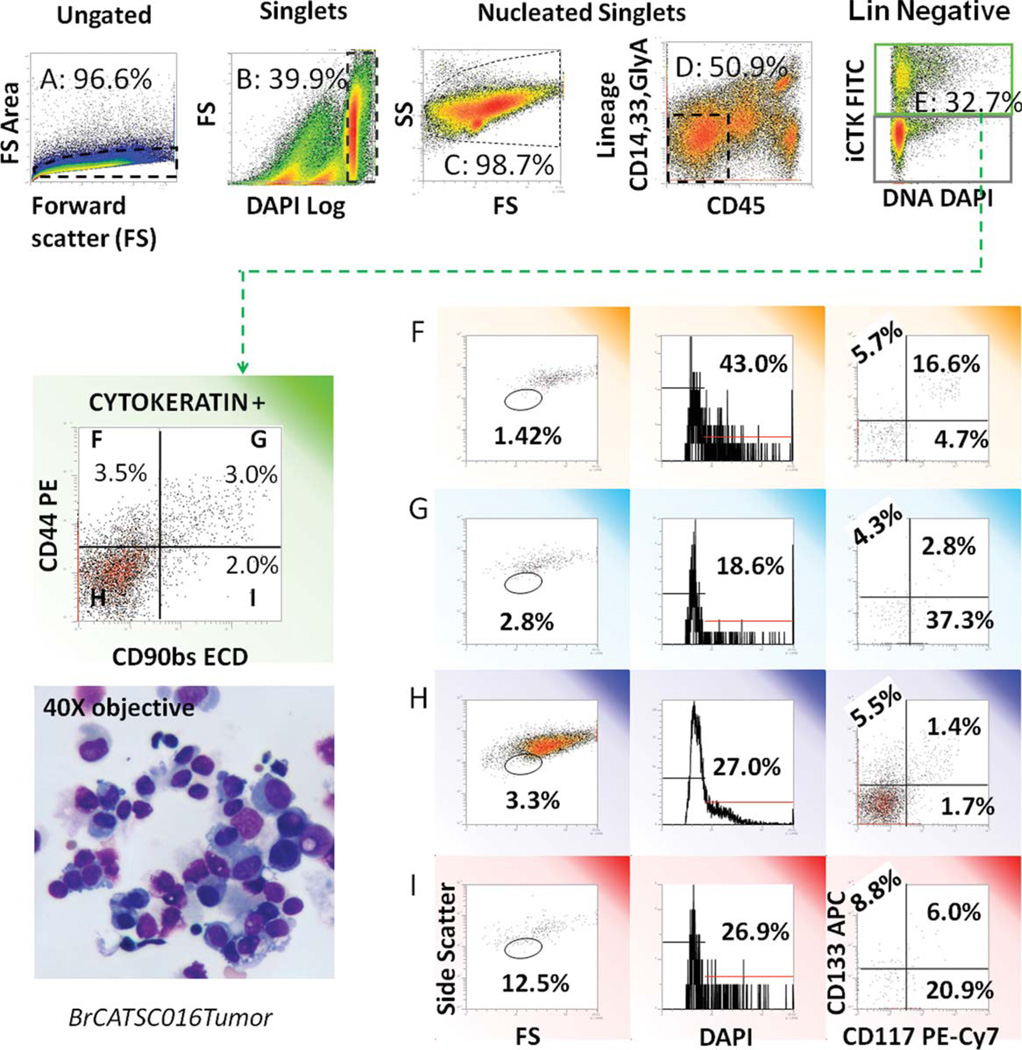
Fig. 2.
Analytical strategy for evaluation of light scatter, DNA content, and stem/progenitor markers in cytokeratin+ nonhematopoietic (non-Heme) tumor cells. A freshly excised tumor was disaggregated and prepared for flow cytometry as described. Five-hundred thousand events were acquired. Top panels: the gating strategy used to remove cell clusters, debris, red, and white blood cells is shown. From left to right: forward light scatter pulse analysis is used to eliminate cell clusters and retain singlet cells (A); the DNA stain DAPI is used to define nucleated cells with ≥2N DNA and eliminate subcellular debris (B); forward versus side light scatter is shown for total nucleated cells (C); CD45 versus a cocktail of CD14, CD33, and glycophorin A is used to gate out Heme cells (D); CD14+ is also expressed on mesothelial cells, which are present in pleural effusions. Cytokeratin positive epithelial cells are subsequently identified (E). This gating strategy was used for all subsequent analyses. Cytokeratin positive cells were sub-setted on the basis of CD90 and CD44 expression. Cell morphology (as determined by light scatter), DNA content (DAPI), and expression of the stem-cell markers CD117 and CD133 were measured on each CD44/CD90 subset. The inset in the lower left shows a cytocentrifuge preparation of the single-cell suspension prepared for flow cytometry. Apoptotic smudge cells and debris are removed from the flow cytometric analysis by the gating strategy. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]