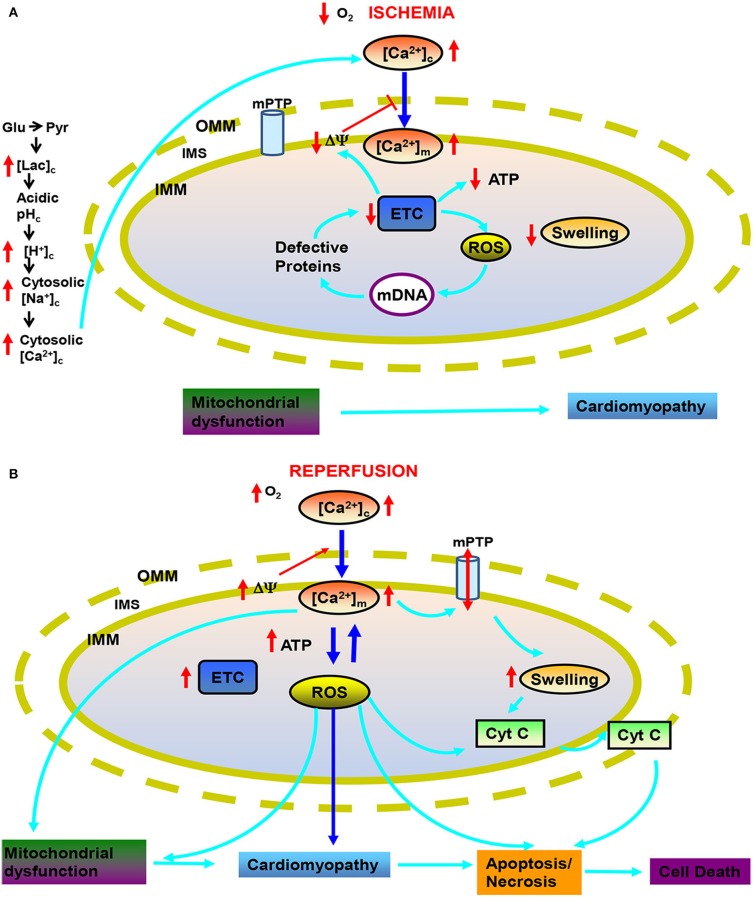
Figure 1.
Targets of mitochondria and sequence of changes in cytosolic and mitochondrial function during cardiac ischemia and reperfusion (IR) injury. During ischemia (A) reduced O2 promotes anaerobic glycolysis that generates increased cytosolic lactate (lacc) leading to acidification. Increased H+ activates Na+-H+ exchanger (NHE) leading to increase cytosolic Na+ ([Na+]c), which activates Na+-Ca2+ exchanger (NCE), causing an increase in cytosolic Ca2+ ([Ca2+]c) which in turn increases mitochondrial matrix Ca2+ ([Ca2+]m). Impaired electron transport leads to increased generation of reactive oxygen species (ROS) beginning with superoxide (O·−2); impaired respiration and substrate utilization leads to uncoupling with lowered mitochondrial membrane potential (ΔΨm) and decreased generation of mitochondrial ATP. During reperfusion (B), the increase in deleterious ROS damages major macromolecules including tricarboxlic acid (TCA) enzymes, membrane transporters, electron transport chain (ETC) proteins and mitochondrial DNA (mtDNA). Also during reperfusion, ΔΨm is restored and [Ca2+]m and ROS further increase to produce even greater mitochondria damage that induces mitochondrial permeability transition pore (mPTP) opening and release of cytochrome c (cyt C) that in turn triggers apoptosis. Other abbreviations: OMM, outer mitochondrial membrane; IMM, inter mitochondrial membrane; IMS, inter mitochondrial space.