Figure 4.
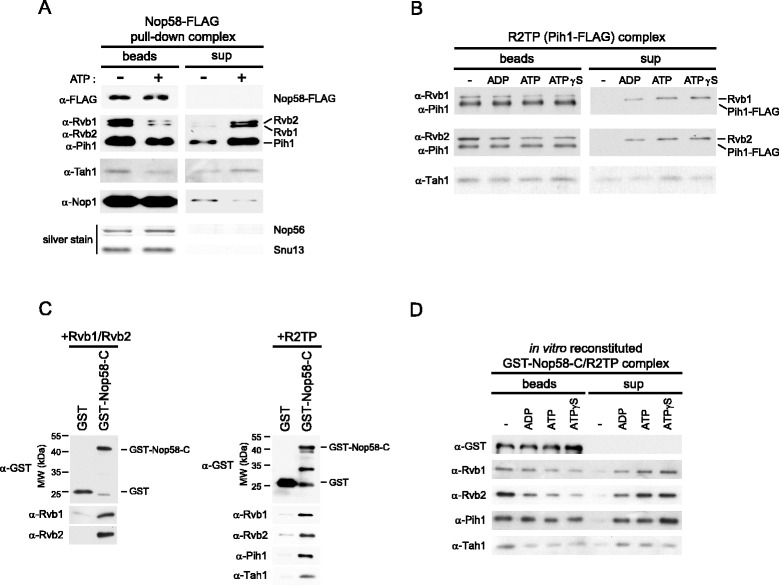
Effect of nucleotides on the interaction between Nop58 and R2TP. (A) Western blot or silver stain analysis of Nop58-FLAG pulldown complex in the absence or presence of ATP. The Nop58-FLAG complex purified on α-FLAG beads from cell lysates was incubated with 4 mM ATP for 30 min at 30°C. Supernatant and bead-bound fractions were, subsequently, analyzed. Nop58-FLAG, Rvb1, Rvb2, Pih1, Tah1, and Nop1 were detected by Western blot, while Nop56 and Snu13 were detected by silver stain (and identified by mass spectrometry). (B) Western blot analysis of the R2TP (Pih1-FLAG) complex in the absence or presence of different nucleotides. The R2TP complex purified on α-FLAG beads from cells having Pih1 endogenously FLAG-tagged was incubated in the absence or presence of 4 mM ADP, ATP, or ATPγS for 30 min at 30°C. Supernatant and bead-bound fractions were, subsequently, analyzed. (C) In vitro binding assay for Nop58 C-terminal domain with Rvb1/2 or R2TP complex. GST alone or GST-Nop58-C (residues 285–447) were bound to glutathione-beads and incubated with Rvb1/2 or R2TP complex. After washing the beads, proteins retained on the beads were detected by Western blot analysis. (D) Western blot analysis of in vitro reconstituted GST-Nop58-C/R2TP complex in the absence or presence of different nucleotides. The GST-Nop58-C/R2TP complex bound to beads prepared as in Figure 4C (right panel) was incubated in the absence or presence of 5 mM ADP, ATP, or ATPγS for 30 min at 30°C. Supernatant and bead-bound fractions were, subsequently, analyzed.
