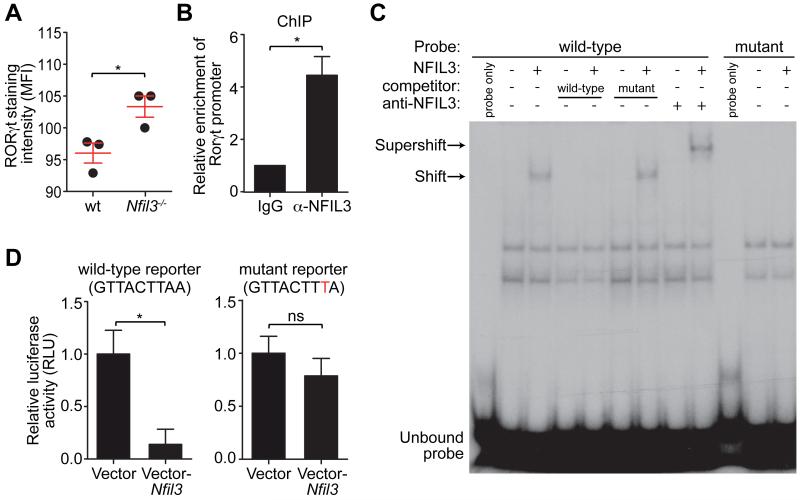
Figure 2. NFIL3 represses Rorγt transcription by binding directly to its promoter.
(A) LPLs from wild-type and Nfil3−/− mice were analyzed by nuclear staining of RORγt and mean fluorescence intensities (MFI) were plotted. (B) ChIP analysis of CD4+ T cells using IgG or anti-NFIL3 antibody. Enrichment of the Rorγt promoter was calculated as the ratio of the anti-NFIL3 to the IgG control pull-down. (C) EMSA with nuclear extracts of HEK293T cells transfected with an empty vector or an NFIL3-encoding vector. A 30-bp DNA fragment encompassing the NFIL3-binding site from the Rorγt promoter was synthesized as a wild-type probe. The mutant probe has the same sequence except that the NFIL3 binding site was mutated. NFIL3 binding specificity was demonstrated by competition with non-radioactively labeled probes and supershift with the anti-NFIL3 antibody. (D) Luciferase reporter assay. A 1018 bp (from −1013 to +5) fragment of the Rorγt promoter was fused with firefly luciferase and position 8 of the NFIL3 binding site was mutated from A to T in the mutant reporter. Jurkat T cells were transfected with reporters and an empty vector or an NFIL3-encoding vector. Luciferase activity was normalized to cells transfected with vector-only controls. Groups were plotted as mean ± SEM and compared by two-tailed student’s t-test. *,p<0.05; ns, not significant.