Figure 7.
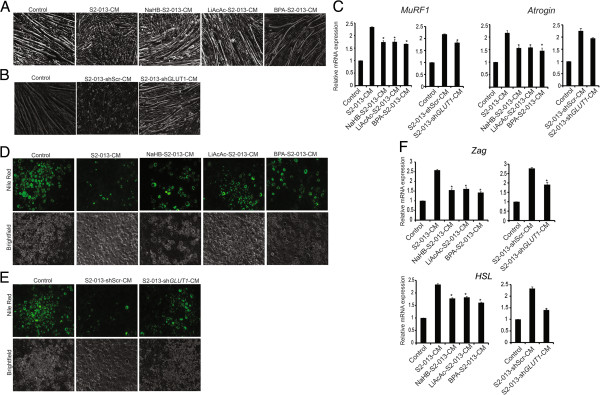
Pretreatment of tumor cells with ketone bodies or glycolytic inhibition diminishes their cachectic potential. S2-013 cells were treated with solvent control, 20 mM NaHB (NaHB-S2-013), 20 mM LiAcAc (LiAcAc-S2-013), and 10 μM 3-bromopyruvic acid (BPA-S2-013) for 24 h. The cells were then washed twice with phosphate-buffered saline and cultured in serum-free DMEM. After 24 h, the conditioned medium was collected. The conditioned medium was also prepared from GLUT1 knockdown S2-013 (S2-013-shGLUT1) and control cells (S2-013-shScr). Differentiated myotubes from C2C12 cells were cultured in (A) control, S2-013-CM, NaHB-S2-013-CM, LiAcAc-S2-013-CM, and BPA-S2-013-CM or (B) control, S2-013-shScr-CM, and S2-013-shGLUT1-CM for 72 h, and bright-field images were represented for individual treatments. Differentiated 3T3L1 cells were cultured in (C) control, S2-013-CM, NaHB-S2-013-CM, LiAcAc-S2-013-CM, and BPA-S2-013-CM or (D) control, S2-013-shScr-CM, and S2-013-shGLUT1-CM for 72 h and stained with nile red, and images for individual treatments are represented. (E) Differentiated myotube form C2C12 cells were cultured in similar conditions for 24 h. Total RNA was isolated and relative mRNA levels of MuRF1 and Atrogin were determined by qRT-PCR. β-Actin was utilized as an internal control. (F) Differentiated 3T3L1 cells were cultured in the above-mentioned conditions for 24 h. Total RNA was isolated and relative mRNA levels of Zag and HSL were determined by qRT-PCR. β-Actin was utilized as an internal control. Values represented are mean ± SEM. All statistical analyses were conducted with one-way ANOVA with Dunnett’s post hoc test and S2-013-CM as the reference group.*P < 0.05; **P < 0.01.
