FIGURE 2.
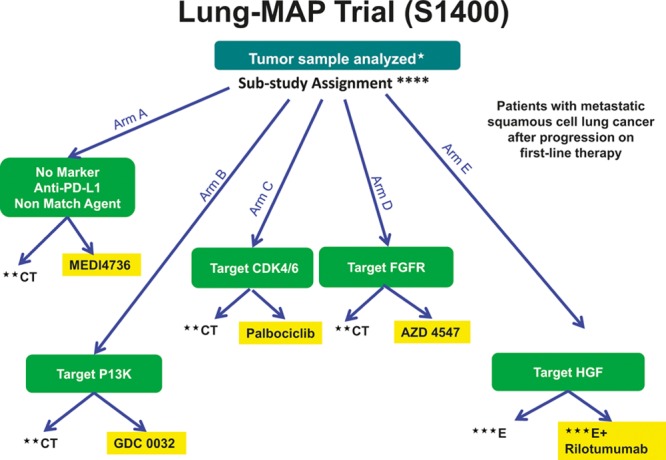
Phase II/III biomarker-driven master protocol for second-line therapy of squamous cell lung cancer (Lung-MAP Trial). *Archival formalin-fixed paraffin-embedded (FFPE) tumor, fresh core needle biopsy if needed. **CT = chemotherapy (docetaxel) control arm. ***E = erlotinib control arm. ****Substudy assignment will be determined based on randomization for patients eligible for multiple substudies.
