Abstract
Mutually exclusive selection of one exon in a cluster of exons is a rare form of alternative pre-mRNA splicing, yet suggests strict regulation. However, the repertoires of regulation mechanisms for the mutually exclusive (ME) splicing in vivo are still unknown. Here, we experimentally explore putative ME exons in C. elegans to demonstrate that 29 ME exon clusters in 27 genes are actually selected in a mutually exclusive manner. Twenty-two of the clusters consist of homologous ME exons. Five clusters have too short intervening introns to be excised between the ME exons. Fidelity of ME splicing relies at least in part on nonsense-mediated mRNA decay for 14 clusters. These results thus characterize all the repertoires of ME splicing in this organism.
Keywords: NMD, alternative splicing, intron, mutually exclusive exons, pre-mRNA processing, proteome diversity
Introduction
Alternative processing of precursor mRNAs (pre-mRNAs) is a major source of protein diversity and plays crucial roles in development, differentiation, and diseases in higher eukaryotes.1-4 One of the most elaborately regulated forms of alternative pre-mRNA processing is mutually exclusive (ME) alternative splicing, through which only one exon is mutually exclusively selected from a cluster of exons at a time to determine critical aspects of the target genes such as ligand-binding specificity of receptors and properties of enzymes and channels.5-7 The ME exons occur only as pairs in vertebrates, but the number of ME exons in a cluster in invertebrates can be more than two in some genes. The extreme example is the Dscam gene of a fruit fly Drosophila melanogaster, which has four ME exon clusters containing 12, 48, 33, and two variants.8
Several mechanisms have been proposed for the mutually exclusive nature of the ME exon selection.9-12 Steric interference between two splice sites of an intervening intron between two ME exons has been proposed to make the ME exons physically incapable of being spliced to each other in some mammalian genes.9,12,13 This is due to a shorter distance between the 5′ splice site and the branch point on the intron than minimal spacing required for a spliceosome to be productively assembled. Another mechanism proposed to prohibit double inclusion and double skipping of the ME exons is spliceosome incompatibility.9,12 This is proposed for a tandem exon pair flanked by U2- and U12-type introns on either side of the exons.14 Disposal of aberrantly spliced mRNAs by a surveillance system termed nonsense-mediated mRNA decay (NMD) is considered to play substantial roles for some genes.15-17 Antagonism of repression by base-paring interaction of a docker with one of the selector sequences is proposed for the Dscam exon 6 cluster.18,19
In C. elegans, it is estimated by a recent genome-wide analysis that up to 25% of the protein-coding genes undergo alternative pre-mRNA processing and 55 events were assigned to ME alternative splicing.20 In previous studies, we have elucidated tissue-specific and/or developmental selection patterns and regulation mechanisms for some of the ME exon clusters in C. elegans by generating fluorescence alternative splicing reporters and isolating splicing factor mutants. In the case of exons 5B/5A of the egl-15 gene, encoding fibroblast growth factor receptors (FGFRs),21-23 the RBFOX family and SUP-12 cooperatively bind to the upstream flanking intron of the upstream exon to repress the upstream exon in muscles. In the case of exons 9/10 of the let-2 gene, encoding α2 subunit of collagen type IV,24 ASD-2 binds to the downstream flanking intron of the downstream exon to promote inclusion of the downstream exon in muscle-specific and developmentally regulated manners.25,26 In the case of exons 7a/7b of the unc-32 gene, encoding subunit a of V0 domain of vacuolar proton-translocating ATPase (V-ATPase),27 UNC-75 binds to the intervening intron to repress the downstream exon and the RBFOX family binds to the downstream intron to promote inclusion of the upstream exon in the nervous system.28 Thus, the tissue-specificity, trans-acting factors, positions of the cis-elements, and functions of the factors for the regulation of ME exon clusters, vary from gene to gene in C. elegans. These findings raise questions about to what extent the repertoires and regulation mechanisms for the ME exon clusters have evolved in this organism.
Here we explore all the 55 putative ME splicing events in C. elegans listed in Ramani et al.20 that utilized high-throughput sequencing and microarray profiling of polyA+ RNAs isolated from four and five different developmental stages, respectively. We experimentally test whether the putative ME exons are actually mutually exclusively selected by reverse transcription-polymerase chain reaction (RT-PCR). For the verified ME exons, we analyze the nucleotide and amino acid sequence identities of the ME exons in each cluster. To also elucidate to what extent the mutually exclusive nature of the exon selection rely on NMD, we compare the RT-PCR patterns between a wild-type strain N2 and an NMD-deficient mutant smg-2.23,29
Results and Discussion
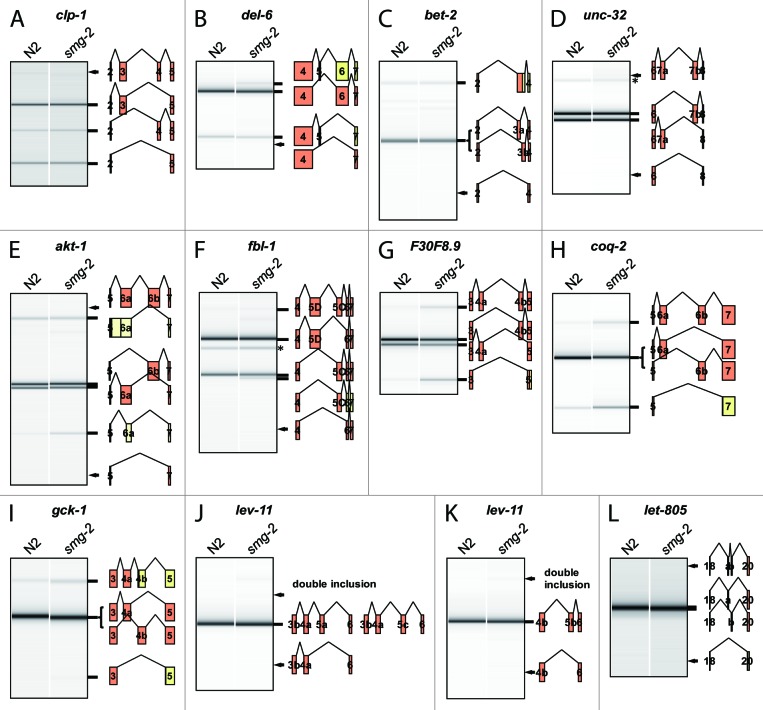
Table 1 summarizes the results of the comprehensive RT-PCR analyses at the L1 stage. The 55 events were assigned to 41 clusters in 37 genes. Eight of the clusters were considered to be tandem cassette exon pairs rather than ME exons because we detected in-frame double-inclusion and/or double-skipping isoforms (> 5% of the sum in molar concentration) in addition to the single-inclusion isoforms (Table 1; Fig. 1A; and data not shown). Notably, these cassette exons are multiple of three (3n) nucleotides (nt) in length except for those carrying natural termination codons (Table 1). Two exons in two genes were considered to be single cassette exons and two other exons in two genes appear to be constitutively included. The other 29 clusters in 27 genes were considered to be mutually exclusive (Table 1) since the single-inclusion isoforms were detected in our experiments and/or in the literature and other isoforms were almost undetectable or degraded by NMD in the wild-type background (see below). We confirmed that the single-inclusion isoforms were also almost exclusively expressed at the young adult stage (data not shown).
Table 1. Summary of experimental validation of the putative ME exon clusters.
| Name and ID in WS239 | Position in WS235 | Supporting Reads*a | Sequence Identity | Intervening Intron(s) [nt]*b | NMD- Dependence*c |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AS Type | Chr | Gene | WBGene ID | Str | Exon | Left end | Right end | Exon Length | Nucleotide | Amino Acid | ||||
| Homologous ME | I | F30F8.9 | WBGene00009276 | + | 4a | 7837844 | 7837926 | 83 | 3n +2 | 9 | 41.0% | 17.9% | 683 | Yes |
| 4b | 7838610 | 7838692 | 83 | 3n +2 | 12 | |||||||||
| lev-11 | WBGene00002978 | - | 7b | 14623010 | 14623148 | 139 | 3n +1 | 1615 | 71.2% | 78.3% | 422 | No | ||
| 7a*d | 14623571 | 14623709 | 139 | 3n +1 | 83 | |||||||||
| 5b | 14624030 | 14624147 | 118 | 3n +1 | 478 | 81.4% | 74.4% | 155, 123 | No | |||||
| 5c | 14624303 | 14624420 | 118 | 3n +1 | 94 | 47.5% | 35.9% | |||||||
| 5a | 14624544 | 14624661 | 118 | 3n +1 | 706 | 83.9% | 71.8% | |||||||
| II | snt-1 | WBGene00004921 | + | 6B*d | 6815048 | 6815187 | 140 | 3n +2 | 0 | 64.3% | 55.3% | 116 | No | |
| 6A | 6815304 | 6815437 | 134 | 3n +2 | 63 | |||||||||
| lat-1 | WBGene00002251 | + | 3a | 8903388 | 8903494 | 107 | 3n +2 | 24 | 55.8% | 45.9% | 112 | Partially | ||
| 3b | 8903607 | 8903719 | 113 | 3n +2 | 72 | |||||||||
| III | let-805 | WBGene00002915 | + | 19a | 2676547 | 2676664 | 118 | 3n +1 | 44 | 40.4% | 33.3% | 97 | No | |
| 19b | 2676762 | 2676870 | 109 | 3n +1 | 34 | |||||||||
| gly-6 | WBGene00001631 | - | 8b | 4325419 | 4325523 | 105 | 3n | 26 | 44.1% | 28.9% | 11 | - | ||
| 8a | 4325535 | 4325642 | 108 | 3n | 49 | |||||||||
| pdfr-1 | WBGene00015735 | - | 8b | 6636402 | 6636556 | 155 | 3n +2 | 77 | 53.4% | 30.8% | 336 | Partially | ||
| 8a | 6636893 | 6637017 | 125 | 3n +2 | 24 | |||||||||
| coq-2 | WBGene00000762 | + | 6a | 6937600 | 6937733 | 134 | 3n +2 | 13 | 47.0% | 40.0% | 678 | Yes | ||
| 6b | 6938412 | 6938545 | 134*e | 3n +2 | 12 | |||||||||
| unc-32 | WBGene00006768 | + | 7a | 8909233 | 8909355 | 123 | 3n | 46 | 47.2% | 35.4% | 725 | - | ||
| 7b | 8910081 | 8910221 | 141 | 3n | 56 | |||||||||
| gly-5 | WBGene00001630 | + | 9b | 13200771 | 13200881 | 111 | 3n | 16 | 77.7% | 54.1% | 34, 1466 | - | ||
| 9c | 13200916 | 13201020 | 105 | 3n | 13 | 66.1% | 54.1% | |||||||
| 9a | 13202487 | 13202588 | 102 | 3n | 6 | 71.4% | 48.6% | |||||||
| W06F12.2 | WBGene00012306 | - | 2b | 13730072 | 13730243 | 172 | 3n +1 | 80 | 35.5% | 17.5% | 129 | Yes | ||
| 2a | 13730373 | 13730526 | 154 | 3n +1 | 28 | |||||||||
| IV | gba-3 | WBGene00008706 | + | 6a | 17478400 | 17478647 | 248 | 3n +2 | 78 | 75.4% | 80.5% | 207 | Yes | |
| 6b | 17478855 | 17479102 | 248 | 3n +2 | 82 | |||||||||
| V | unc-62 | WBGene00006796 | + | 7a*d | 4504975 | 4505120 | 146 | 3n +2 | 4 | 49.1% | 30.2% | 238 | No | |
| 7b | 4505359 | 4505516 | 158 | 3n +2 | 162 | |||||||||
| gck-1 | WBGene00001526 | + | 4a | 8952725 | 8952879 | 155 | 3n +2 | 19 | 52.2% | 48.1% | 138 | Partially | ||
| 4b | 8953018 | 8953175 | 158 | 3n +2 | 31 | |||||||||
| akt-1 | WBGene00000102 | + | 6a | 10250841 | 10251032 | 192 | 3n | 136 | 62.8% | 63.8% | 294 | - | ||
| 6b | 10251327 | 10251533 | 207 | 3n | 41 | |||||||||
| atn-1 | WBGene00000228 | + | 4a | 12480728 | 12480888 | 161 | 3n +2 | 147 | 61.4% | 57.1% | 225 | Partially | ||
| 4b | 12481114 | 12481196 | 83 | 3n +2 | 26 | |||||||||
| slo-1 | WBGene00004830 | + | 10a | 18499582 | 18499694 | 113 | 3n +2 | 21 | 65.5% | 64.1% | 856 | Partially | ||
| 10b | 18500551 | 18500663 | 113 | 3n +2 | 11 | |||||||||
| X | mrp-1 | WBGene00003407 | - | 13D | 579498 | 579653 | 156 | 3n | 31*f | 68.6% | 53.8% | 334, 154, 435 | - | |
| 13C | 579988 | 580143 | 156 | 3n | 35 | 76.9% | 59.6% | |||||||
| 13B | 580298 | 580453 | 156 | 3n | 37 | 74.4% | 53.8% | |||||||
| 13A | 580889 | 581044 | 156 | 3n | 2 | 69.2% | 50.0% | |||||||
| cca-1 | WBGene00000367 | - | 18b | 7854051 | 7854201 | 151 | 3n +1 | 8 | 53.0% | 44.0% | 97 | Partially | ||
| 18a | 7854299 | 7854428 | 130 | 3n +1 | 20 | |||||||||
| bet-2 | WBGene00010199 | + | 3a | 10571708 | 10571827 | 120 | 3n | 35 | 45.8% | 35.0% | 37 | - | ||
| 3b | 10571865 | 10571984 | 120 | 3n | 32 | |||||||||
| let-2 | WBGene00002280 | - | 10 | 16386613 | 16386723 | 111 | 3n | 164 | 65.8% | 56.8% | 30 | - | ||
| 9 | 16386754 | 16386861 | 108 | 3n | 104 | |||||||||
| Non-Homologous ME | I | pqn-72 | WBGene00004154 | - | 6 | 3274267 | 3274812 | 546 | 3n | 53 | 37.0% | 21.6% | 117 | - |
| 5 | 3274930 | 3275010 | 81 | 3n | 3 | |||||||||
| tom-1 | WBGene00006594 | + | 14a | 5552880 | 5553369 | 490 | 3n +1 | 172 | 50.0% | 16.4% | 1371 | Partially | ||
| 14b | 5554741 | 5554864 | 124 | 3n +1 | 72 | |||||||||
| ttx-7 | WBGene00008765 | - | 5b | 7301663 | 7301774 | 112 | 3n +1 | 16 | 41.8% | 17.1% | 132 | Partially | ||
| 5a | 7301907 | 7302027 | 121 | 3n +1 | 54 | |||||||||
| III | unc-32 | WBGene00006768 | + | 4a | 8906524 | 8906678 | 155 | 3n +2 | 34 | 60.0% | 17.3% | 288, 237 | Partially | |
| 4b | 8906967 | 8907073 | 107 | 3n +2 | 49 | 51.6% | 18.0% | |||||||
| 4c | 8907311 | 8907432 | 122 | 3n +2 | 26 | 49.7% | 23.1% | |||||||
| IV | fbl-1 | WBGene00001403 | + | 5D | 9542035 | 9542292 | 258 | 3n | 57 | 35.0% | 22.1% | 435 | - | |
| 5C | 9542728 | 9542868 | 141 | 3n | 10 | |||||||||
| V | del-6 | WBGene00011891 | + | 5 | 10574706 | 10574733 | 28 | 3n +1*g | 4 | 53%*h | 60%*h | 200 | Yes | |
| 6 | 10574934 | 10575098 | 165 | 3n | 230 | |||||||||
| X | egl-15 | WBGene00001184 | + | 5B | 11017580 | 11017930 | 351 | 3n | 38 | 40.1% | 10.3% | 14 | - | |
| 5A | 11017945 | 11018139 | 195 | 3n | 5 | |||||||||
| Tandem CE | I | tom-1 | WBGene00006594 | + | 17 | 5557960 | 5557971 | 12 | 3n | - | - | - | - | - |
| Tandem CE | 18 | 5558453 | 5558479 | 27 | 3n | - | - | - | - | - | ||||
| Tandem CE | I | lev-11 | WBGene00002978 | - | 9b | 14622304 | 14622511 | 208 | 3n +1*g | - | - | - | - | - |
| Tandem CE | 9a | 14622658 | 14622743 | 86 | 3n +2*g | - | - | - | - | - | ||||
| Single CE | II | etr-1 | WBGene00001340 | + | 4 | 166071 | 166150 | 80 | 3n +2 | - | - | - | - | - |
| Tandem CE | II | zyg-12 | WBGene00006997 | + | 8 | 4952490 | 4952531 | 42 | 3n*g | - | - | - | - | - |
| Tandem CE | 9 | 4952647 | 4952694 | 48 | 3n | - | - | - | - | - | ||||
| Tandem CE | II | C34F11.3 | WBGene00016415 | + | 10 | 5205040 | 5205075 | 36 | 3n | - | - | - | - | - |
| Tandem CE | 11 | 5205444 | 5205632 | 189 | 3n | - | - | - | - | - | ||||
| Tandem CE | III | clp-1 | WBGene00000542 | + | 3 | 7981673 | 7981810 | 138 | 3n | - | - | - | - | - |
| Tandem CE | 4 | 7982464 | 7982538 | 75 | 3n | - | - | - | - | - | ||||
| Constitutive | IV | unc-44 | WBGene00006780 | + | 13 | 5984796 | 5984980 | 185 | 3n +2 | - | - | - | - | - |
| Single CE | 14 | 5985093 | 5985170 | 78 | 3n | - | - | - | - | - | ||||
| Undetected | 15 | 5985577 | 5986355 | 779 | 3n +2 | - | - | - | - | - | ||||
| Alternative Acceptors | IV | unc-43 | WBGene00006779 | - | 14S | 10329097 | 10329195 | 99*i | 3n | - | - | - | - | - |
| Tandem CE | V | K10D6.2 | WBGene00010742 | - | 5L | 11194203 | 11194342 | 140*j | 3n +2*g | - | - | - | - | - |
| Tandem CE | 4 | 11194574 | 11194627 | 54 | 3n | - | - | - | - | - | ||||
| Tandem CE | V | Y69H2.3 | WBGene00013481 | - | 7 | 18661622 | 18661711 | 90 | 3n | - | - | - | - | - |
| Tandem CE | 6 | 18662119 | 18662313 | 195 | 3n | - | - | - | - | - | ||||
| Constitutive | X | F49E2.5 | WBGene00009888 | + | 5 | 9554299 | 9554547 | 249 | 3n | - | - | - | - | - |
| Tandem CE | 6 | 9554692 | 9554823 | 132 | 3n | - | - | - | - | - | ||||
| Constitutive | X | T23E7.2 | WBGene00020732 | + | 16 | 17679922 | 17680035 | 114 | 3n | - | - | - | - | - |
ME, mutually exclusive exons; CE, cassette exon. *aTotal number of sequence reads mapped to each ME exon and its junctions with the flanking exons out of 10.3 million mapped reads in RNA-seq analysis of polyA+ RNA from synchronized smg-2 L1 larvae.43 *bIntervening introns shorter than 40 nt are underlined. *cYes, NMD isoform(s) were significantly increased in the smg-2 mutant (P < 0.05 in a modified χ2 test of the RT-PCR products43). Partially, NMD isoform(s) were slightly increased in the smg-2 mutant (difference in the amount of the splice variant > 0.4 [% of sum] in molar concentration). No, no apparent difference in the RT-PCR patterns between N2 and the smg-2 mutant. *dThese exons were expressed at very low levels at the L1 stage. At the young adult stage, lev-11 exon 7a and snt-1 exon 6B were still rare, while unc-62 exon 7a was readily detected. *eOut-of-frame tandem acceptor sites were frequently used for coq-2 exon 6b. *fmrp-1 exon 13D is aberrantly annotated in the RefSeq model used for mapping. *gThese exons carry in-frame termination codons. *hNucleotide and amino acid sequence identities of del-6 exons 5/6 appear high because of their short lengths. *iIn-frame tandem acceptor sites were frequently used for unc-43 exon 14. *jTandem donor sites were frequently used for K10D6.2 exon 5.
Figure 1. RT-PCR analyses of the putative ME exons in the wild-type (N2) and the smg-2 (yb979) mutant. (A) clp-1 exons 3/4. (B) del-6 exons 5/6. del-6 exon 5 is unique in that it carries a natural termination codon. (C) bet-2 exons 3a/3b. Note that the intervening intron is retained instead of double ME exon inclusion for this cluster. (D) unc-32 exons 7a/7b.28 (E) akt-1 exons 6a/6b. A non-productive exon 6a isoform utilizing an aberrant acceptor site is detected in the smg-2 mutant. (F) fbl-1 exons 5D/5C.50 (G) F30F8.9 exons 4a/4b. (H) coq-2 exons 6a/6b. (I) gck-1 exons 4a/4b. (J and K) lev-11 exons 5a/5c/5b.37 (L) let-805 exons 19a/19b. Splicing patterns are schematically indicated. Coding regions are in orange. Arrows indicate predicted positions of undetected isoforms indicated on the right. Asterisks indicate non-specific bands.
Features of the ME exon clusters
The 29 ME exon clusters can be divided into two groups according to sequence similarity of the ME exons. Homologous ME exon clusters include 19 pairs, two trios, and one quad of homologous ME exons, while non-homologous clusters include six pairs and one trio of non-homologous ME exons (Table 1). The homologous clusters may be originated from exon duplication.14,30 The lengths of the homologous ME exons are close to or exactly the same as the counterpart(s) except for atn-1 exons 4a/4b, while those of the non-homologous ME exons are often far different from the counterpart(s). Nevertheless, reading frames in the downstream common exons are preserved whichever exon in the clusters is selected in almost all cases. The only exception is exons 5/6 of the del-6 gene, encoding a degenerin-like ion channel protein, where exon 5 consists of 3n+1 nt and carries a natural termination codon while exon 6 consists of 3n nt and has no termination codon (Fig. 1B).
Four of the ME exon clusters consist of more than two ME exons. Exons 13A/13B/13C/13D of the mrp-1 gene, encoding an ATP-binding cassette (ABC) transporter,31 is the only cluster with four ME exons. All the four exons are 156 (3n) nt in length and are homologous to each other (Table 1). Exons 9b/9c/9a of the gly-5 gene, encoding a UDP-GalNAc:polypeptide N-acetylgalactosaminyltransferase,32 is one of the three clusters with three ME exons. These exons are homologous to each other with almost the same size of 3n nt. The intervening intron between exons 9b and 9c is just 34 nt (discussed later). Exons 4a/4b/4c of the unc-32 gene27 are the only non-homologous trio of the ME exons. The unc-32 gene has another cluster of ME exons 7a/7b (Table 1) and we have recently reported that these two clusters in the single gene are independently regulated in tissue-specific manners.28 Exons 5a/5c/5b of the lev-11 gene, encoding multiple tropomyosin isoforms,33 are homologous and of exactly the same size (3n+1 nt). Pre-mRNA processing of the lev-11 gene is complex due to the combination of tissue-specific promoters, clusters of ME exons 4a/4b, 5a/5b/5c, and 7a/7b, and tandem cassette exons 9a/9b (Table 1).33 The complex structures and pre-mRNA processing patterns of the tropomyosin genes are evolutionarily conserved in metazoans,34 suggesting functional significance of multiple tropomyosin isoforms.
Steric interference to prohibit double inclusion of ME exons
Size distribution of the overall introns suggests that the minimal size of the introns is ~40 nt in C. elegans.35 Among the ME exon clusters we have already reported, the intervening introns for exons 5B/5A of the egl-15 gene21-23 and exons 9/10 of the let-2 gene24,25 are 14 nt and 30 nt, respectively, and we have never observed mRNA isoforms where these short introns are excised. These observations are consistent with the idea that the short introns of less than 40 nt cannot be excised because of the steric interference like in mammals, although no strong consensus are found for the branch point in C. elegans. According to this criterion, three more clusters are considered to be physically incapable of double exon inclusion: exons 3a/3b of bet-2 encoding a BET (two bromodomains) family protein (Fig. 1C), gly-5 exons 9b/9c, and exons 8a/8b of gly-6, a paralog of gly-5 (Table 1).32 Notably, the lengths of all the ME exons in these five clusters are 3n nt.
As U11 or U12 snRNA or an AT/AC splice-junction are not found in C. elegans,36 we need not consider the spliceosome incompatibility in the regulation of the ME exons here.
NMD-dependence of the mutually exclusive selection
If the lengths of the ME exons are 3n nt, inclusion or skipping of the ME exons does not cause a frame-shift or a premature termination codon (PTC) in the mRNA isoforms. Consistent with this idea, there was no apparent difference in the amounts of multiple-inclusion and all-skipping isoforms between the wild-type and the smg-2 mutant for such clusters (Table 1; Fig. 1D–F; and data not shown).
If the lengths of the ME exons are not 3n nt, multiple inclusion and all-skipping of the ME exons cause frame-shifts to create aberrant termination codons and such mRNA isoforms should be eliminated by NMD. We found that multiple-inclusion and/or all-skipping isoforms are evidently or slightly more abundant in the smg-2 mutant than in the wild-type for 14 out of the 19 clusters where the ME exons are not 3n nt (Table 1; Fig. 1G–I; and data not shown), indicating that these non-productive mRNA isoforms are actually eliminated by NMD in the wild-type. For lev-11 exons 7a/7b,37 snt-1 exons 6B/6A,38 and unc-62 exons 7a/7b,39 we confirmed predominant use of only one of the two ME exons (Table 1) as in the literature and this can be a reason why aberrantly spliced isoforms are rare and undetectable for these clusters. For the other two clusters, lev-11 exons 5a/5c/5b37 and let-805 exons 19a/19b, the RT-PCR patterns were indistinguishable between the wild-type and the smg-2 mutant (Table 1; Fig. 1J–L). All these results indicate that the fidelity of the splicing regulation varies among the ME exon clusters and some of them rely on the mRNA surveillance system.
Statistics of the ME exons and flanking introns
Figure 2 summarizes the statistics of the 63 experimentally verified ME exons and their flanking and intervening introns.
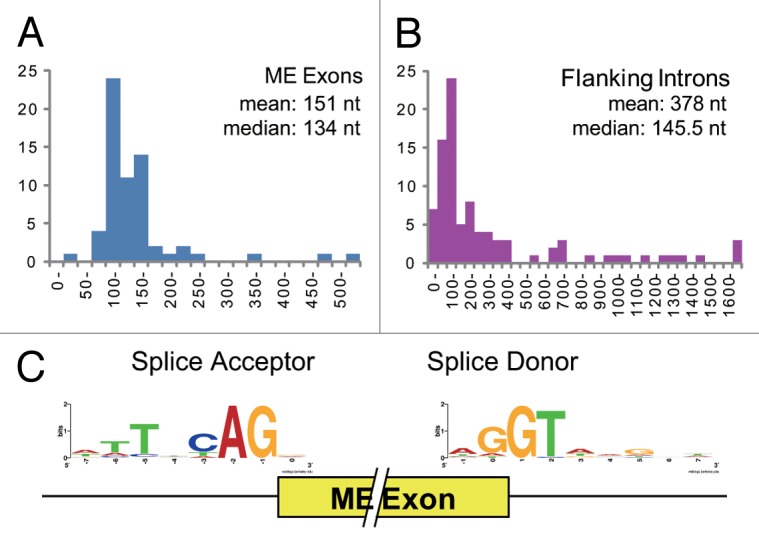
Figure 2. Statistics of the ME exons and their flanking introns. (A and B) Size distributions of the 63 verified ME exons (A) and their 92 flanking introns, including the intervening introns (B). The mean and median sizes are also indicated. (C) Sequence logos of the splice acceptor and donors sites of the 63 ME exons.
The median size of the ME exons (134 nt) (Fig. 2A) is similar to those of the entire unique exons in confirmed genes (144 nt).40 Most of the ME exons (60 of 63) are shorter than 260 nt and the average size (151 nt) is close to the median (Fig. 2A). In contrast, the size distribution of the entire unique exons has a fatter tail,35 making the average of 201 nt.40 The three ME exons longer than 350 nt belong to distinct non-homologous ME exon clusters (Table 1). The shortest ME exon (28 nt) exceptionally carries a natural termination codon (Table 1; Fig. 1B). Therefore, the size of the 48 homologous ME exons are in a relatively narrow range (83–248 nt) for C. elegans.
The mean size of the introns flanking the ME exons, including the five short intervening introns discussed above, is 378 nt (Fig. 2B), substantially longer than the overall average of the introns (267 nt).35 The median size of the introns flanking the ME exons is 145.5 nt (Fig. 2B), whereas more than half of all the C. elegans introns are 100 nt or less and most of them are near the minimal length,35 indicating that the introns flanking the ME exons tend to be longer than constitutive introns. This is consistent with a previous finding that many of cis-elements regulating alternative splicing in C. elegans are found in introns.41 Eleven out of the 29 ME exon clusters have UGCAUG stretch(es) in the flanking introns and/or in the ME exons (data not shown), suggesting tissue-specific splicing regulation by the RBFOX family splicing factors ASD-1 and FOX-1.42 Six out of the 29 clusters are affected in the unc-75 mutant,43 suggesting neuron-specific splicing regulation.
Figure 2C summarizes the sequences of the splice acceptor and donor sites for the verified ME exons. These are more diversified from the consensus sequences of the acceptor site (TTTTCAG/R)44 and the donor site (AG/GTAAGTT)45 in C. elegans, where R stands for A or G. Furthermore, two (2.2%) of the 92 flanking introns, let-2 intron 10 and del-6 intron 6, start with GC, a weaker donor than GT,46,47 although GC-AG introns are rare (0.373%) in C. elegans like in other eukaryotes.45 Therefore, the splice sites of the ME exons are considered to be weaker than those of constitutive exons, consistent with previous findings on alternative splice sites in higher organisms.48
Table 2 summarizes gene ontology (GO) analysis of 25 genes with GO terms out of the 27 genes with the verified ME exon clusters. It indicates enrichment of genes encoding membrane or extracellular matrix proteins (P < 0.001, Fisher’s exact test).
Table 2. Gene ontology analysis of 25 genes with the ME exons and GO terms.
| Ontology type | GO term ID | Fold enrichment | Count in 25 Genes with ME Exons and GO terms | Count in all genes with GO Terms (12,834) | P value (Fisher's Exact Test) | Term |
|---|---|---|---|---|---|---|
| Biological_process | GO:0046928 | 257 | 2 | 4 | 2.18E-05 | regulation of neurotransmitter secretion |
| GO:0030163 | 16 | 4 | 131 | 1.11E-04 | protein catabolic process | |
| GO:0007166 | 79 | 2 | 13 | 2.80E-04 | cell surface receptor linked signal transduction | |
| GO:0040011 | 3.5 | 9 | 1327 | 5.77E-04 | locomotion | |
| GO:0034765 | 49 | 2 | 21 | 7.48E-04 | regulation of ion transmembrane transport | |
| GO:0043050 | 45 | 2 | 23 | 8.99E-04 | pharyngeal pumping | |
| Cellular_component | GO:0005865 | 114 | 2 | 9 | 1.30E-04 | striated muscle thin filament |
| GO:0016021 | 2.9 | 12 | 2143 | 2.77E-04 | integral to membrane | |
| GO:0016020 | 3.1 | 11 | 1847 | 3.37E-04 | membrane | |
| GO:0005604 | 57 | 2 | 18 | 5.47E-04 | basement membrane | |
| GO:0005578 | 47 | 2 | 22 | 8.22E-04 | proteinaceous extracellular matrix | |
| Molecular_function | GO:0005201 | 257 | 2 | 4 | 2.18E-05 | extracellular matrix structural constituent |
| GO:0005244 | 49 | 2 | 21 | 7.48E-04 | voltage-gated ion channel activity |
Conclusion
We demonstrated that the 29 ME exon clusters in the 27 genes are actually regulated in a mutually exclusive manner in C. elegans. Twenty-two of the 29 clusters consist of two to four homologous ME exons. Ten of the 29 clusters consist of ME exons with the lengths of 3n nt, five of which have too short intervening introns to be excised. Fourteen of the 19 clusters with the ME exons other than 3n nt in length rely at least in part on NMD. Nevertheless, many of the ME exon clusters appear to be strictly regulated. Further molecular and functional analyses of such clusters will elucidate novel mechanisms for mutually exclusive selection of the ME exons in vivo.
Materials and Methods
Total RNAs were extracted from synchronized L1 larvae of N2 and KH1668: smg-2 (yb979) I strains as described previously.26 RT-PCR was performed essentially as described previously.26 RT-PCR products were analyzed by using BioAnalyzer (Agilent) as described previously.43 The sequences of the RT-PCR products were confirmed by direct sequencing or by cloning and sequencing. Sequences of the primers used in the RT-PCR assays are available upon request to Kuroyanagi H.
A list of the GO terms was retrieved from the Gene Ontology website (http://www.geneontology.org/). Fisher’s exact test was performed by using Ekuseru-Toukei 2010 (Social Survey Research Information). Sequence logos were generated by using WebLogo349 at http://weblogo.threeplusone.com/create.cgi.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Arun K Ramani and Andrew G Fraser of University of Toronto for detailed information about alternative splicing events in C. elegans. We thank Hiroaki Iwasa of Tokyo Medical and Dental University (TMDU) for fruitful discussion. We thank Caenorhabditis Genetics Center for N2 and a bacterial strain OP50.
Glossary
Abbreviations:
- GO
gene ontology
- ME
mutually exclusive
- NMD
nonsense-mediated mRNA decay
- nt
nucleotide
- pre-mRNA
precursor messenger RNA
- PTC
premature termination codon
- RT-PCR
reverse transcription-polymerase chain reaction
References
- 1.Li Q, Lee JA, Black DL. Neuronal regulation of alternative pre-mRNA splicing. Nat Rev Neurosci. 2007;8:819–31. doi: 10.1038/nrn2237. [DOI] [PubMed] [Google Scholar]
- 2.Wang GS, Cooper TA. Splicing in disease: disruption of the splicing code and the decoding machinery. Nat Rev Genet. 2007;8:749–61. doi: 10.1038/nrg2164. [DOI] [PubMed] [Google Scholar]
- 3.Chen M, Manley JL. Mechanisms of alternative splicing regulation: insights from molecular and genomics approaches. Nat Rev Mol Cell Biol. 2009;10:741–54. doi: 10.1038/nrm2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalsotra A, Cooper TA. Functional consequences of developmentally regulated alternative splicing. Nat Rev Genet. 2011;12:715–29. doi: 10.1038/nrg3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matlin AJ, Clark F, Smith CW. Understanding alternative splicing: towards a cellular code. Nat Rev Mol Cell Biol. 2005;6:386–98. doi: 10.1038/nrm1645. [DOI] [PubMed] [Google Scholar]
- 6.Keren H, Lev-Maor G, Ast G. Alternative splicing and evolution: diversification, exon definition and function. Nat Rev Genet. 2010;11:345–55. doi: 10.1038/nrg2776. [DOI] [PubMed] [Google Scholar]
- 7.David CJ, Chen M, Assanah M, Canoll P, Manley JL. HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature. 2010;463:364–8. doi: 10.1038/nature08697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmucker D, Clemens JC, Shu H, Worby CA, Xiao J, Muda M, Dixon JE, Zipursky SL. Drosophila Dscam is an axon guidance receptor exhibiting extraordinary molecular diversity. Cell. 2000;101:671–84. doi: 10.1016/S0092-8674(00)80878-8. [DOI] [PubMed] [Google Scholar]
- 9.Smith CW. Alternative splicing--when two’s a crowd. Cell. 2005;123:1–3. doi: 10.1016/j.cell.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Takeuchi A, Hosokawa M, Nojima T, Hagiwara M. Splicing reporter mice revealed the evolutionally conserved switching mechanism of tissue-specific alternative exon selection. PLoS One. 2010;5:e10946. doi: 10.1371/journal.pone.0010946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Y, Zhan L, Zhang W, Sun F, Wang W, Tian N, Bi J, Wang H, Shi D, Jiang Y, et al. RNA secondary structure in mutually exclusive splicing. Nat Struct Mol Biol. 2011;18:159–68. doi: 10.1038/nsmb.1959. [DOI] [PubMed] [Google Scholar]
- 12.Pohl M, Bortfeldt RH, Grützmann K, Schuster S. Alternative splicing of mutually exclusive exons--a review. Biosystems. 2013;114:31–8. doi: 10.1016/j.biosystems.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Smith CW, Nadal-Ginard B. Mutually exclusive splicing of alpha-tropomyosin exons enforced by an unusual lariat branch point location: implications for constitutive splicing. Cell. 1989;56:749–58. doi: 10.1016/0092-8674(89)90678-8. [DOI] [PubMed] [Google Scholar]
- 14.Letunic I, Copley RR, Bork P. Common exon duplication in animals and its role in alternative splicing. Hum Mol Genet. 2002;11:1561–7. doi: 10.1093/hmg/11.13.1561. [DOI] [PubMed] [Google Scholar]
- 15.Jones RB, Wang F, Luo Y, Yu C, Jin C, Suzuki T, Kan M, McKeehan WL. The nonsense-mediated decay pathway and mutually exclusive expression of alternatively spliced FGFR2IIIb and -IIIc mRNAs. J Biol Chem. 2001;276:4158–67. doi: 10.1074/jbc.M006151200. [DOI] [PubMed] [Google Scholar]
- 16.Spellman R, Rideau A, Matlin A, Gooding C, Robinson F, McGlincy N, Grellscheid SN, Southby J, Wollerton M, Smith CW. Regulation of alternative splicing by PTB and associated factors. Biochem Soc Trans. 2005;33:457–60. doi: 10.1042/BST0330457. [DOI] [PubMed] [Google Scholar]
- 17.Tang ZZ, Sharma S, Zheng S, Chawla G, Nikolic J, Black DL. Regulation of the mutually exclusive exons 8a and 8 in the CaV1.2 calcium channel transcript by polypyrimidine tract-binding protein. J Biol Chem. 2011;286:10007–16. doi: 10.1074/jbc.M110.208116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graveley BR. Mutually exclusive splicing of the insect Dscam pre-mRNA directed by competing intronic RNA secondary structures. Cell. 2005;123:65–73. doi: 10.1016/j.cell.2005.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olson S, Blanchette M, Park J, Savva Y, Yeo GW, Yeakley JM, Rio DC, Graveley BR. A regulator of Dscam mutually exclusive splicing fidelity. Nat Struct Mol Biol. 2007;14:1134–40. doi: 10.1038/nsmb1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramani AK, Calarco JA, Pan Q, Mavandadi S, Wang Y, Nelson AC, Lee LJ, Morris Q, Blencowe BJ, Zhen M, et al. Genome-wide analysis of alternative splicing in Caenorhabditis elegans. Genome Res. 2011;21:342–8. doi: 10.1101/gr.114645.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodman SJ, Branda CS, Robinson MK, Burdine RD, Stern MJ. Alternative splicing affecting a novel domain in the C. elegans EGL-15 FGF receptor confers functional specificity. Development. 2003;130:3757–66. doi: 10.1242/dev.00604. [DOI] [PubMed] [Google Scholar]
- 22.Kuroyanagi H, Kobayashi T, Mitani S, Hagiwara M. Transgenic alternative-splicing reporters reveal tissue-specific expression profiles and regulation mechanisms in vivo. Nat Methods. 2006;3:909–15. doi: 10.1038/nmeth944. [DOI] [PubMed] [Google Scholar]
- 23.Kuroyanagi H, Ohno G, Mitani S, Hagiwara M. The Fox-1 family and SUP-12 coordinately regulate tissue-specific alternative splicing in vivo. Mol Cell Biol. 2007;27:8612–21. doi: 10.1128/MCB.01508-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sibley MH, Johnson JJ, Mello CC, Kramer JM. Genetic identification, sequence, and alternative splicing of the Caenorhabditis elegans alpha 2(IV) collagen gene. J Cell Biol. 1993;123:255–64. doi: 10.1083/jcb.123.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohno G, Hagiwara M, Kuroyanagi H. STAR family RNA-binding protein ASD-2 regulates developmental switching of mutually exclusive alternative splicing in vivo. Genes Dev. 2008;22:360–74. doi: 10.1101/gad.1620608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuroyanagi H, Ohno G, Sakane H, Maruoka H, Hagiwara M. Visualization and genetic analysis of alternative splicing regulation in vivo using fluorescence reporters in transgenic Caenorhabditis elegans. Nat Protoc. 2010;5:1495–517. doi: 10.1038/nprot.2010.107. [DOI] [PubMed] [Google Scholar]
- 27.Pujol N, Bonnerot C, Ewbank JJ, Kohara Y, Thierry-Mieg D. The Caenorhabditis elegans unc-32 gene encodes alternative forms of a vacuolar ATPase a subunit. J Biol Chem. 2001;276:11913–21. doi: 10.1074/jbc.M009451200. [DOI] [PubMed] [Google Scholar]
- 28.Kuroyanagi H, Watanabe Y, Hagiwara M. CELF family RNA-binding protein UNC-75 regulates two sets of mutually exclusive exons of the unc-32 gene in neuron-specific manners in Caenorhabditis elegans. PLoS Genet. 2013;9:e1003337. doi: 10.1371/journal.pgen.1003337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pulak R, Anderson P. mRNA surveillance by the Caenorhabditis elegans smg genes. Genes Dev. 1993;7:1885–97. doi: 10.1101/gad.7.10.1885. [DOI] [PubMed] [Google Scholar]
- 30.Kondrashov FA, Koonin EV. Origin of alternative splicing by tandem exon duplication. Hum Mol Genet. 2001;10:2661–9. doi: 10.1093/hmg/10.23.2661. [DOI] [PubMed] [Google Scholar]
- 31.Yabe T, Suzuki N, Furukawa T, Ishihara T, Katsura I. Multidrug resistance-associated protein MRP-1 regulates dauer diapause by its export activity in Caenorhabditis elegans. Development. 2005;132:3197–207. doi: 10.1242/dev.01909. [DOI] [PubMed] [Google Scholar]
- 32.Hagen FK, Nehrke K. cDNA cloning and expression of a family of UDP-N-acetyl-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase sequence homologs from Caenorhabditis elegans. J Biol Chem. 1998;273:8268–77. doi: 10.1074/jbc.273.14.8268. [DOI] [PubMed] [Google Scholar]
- 33.Anyanful A, Sakube Y, Takuwa K, Kagawa H. The third and fourth tropomyosin isoforms of Caenorhabditis elegans are expressed in the pharynx and intestines and are essential for development and morphology. J Mol Biol. 2001;313:525–37. doi: 10.1006/jmbi.2001.5052. [DOI] [PubMed] [Google Scholar]
- 34.Irimia M, Maeso I, Gunning PW, Garcia-Fernàndez J, Roy SW. Internal and external paralogy in the evolution of tropomyosin genes in metazoans. Mol Biol Evol. 2010;27:1504–17. doi: 10.1093/molbev/msq018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, et al. International Human Genome Sequencing Consortium Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 36.Stricklin SL, Griffiths-Jones S, Eddy SR., Sr. C. elegans noncoding RNA genes. WormBook. 2005:1–7. doi: 10.1895/wormbook.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kagawa H, Sugimoto K, Matsumoto H, Inoue T, Imadzu H, Takuwa K, Sakube Y. Genome structure, mapping and expression of the tropomyosin gene tmy-1 of Caenorhabditis elegans. J Mol Biol. 1995;251:603–13. doi: 10.1006/jmbi.1995.0459. [DOI] [PubMed] [Google Scholar]
- 38.Mathews EA, Mullen GP, Crowell JA, Duerr JS, McManus JR, Duke A, Gaskin J, Rand JB. Differential expression and function of synaptotagmin 1 isoforms in Caenorhabditis elegans. Mol Cell Neurosci. 2007;34:642–52. doi: 10.1016/j.mcn.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Auken K, Weaver D, Robertson B, Sundaram M, Saldi T, Edgar L, Elling U, Lee M, Boese Q, Wood WB. Roles of the Homothorax/Meis/Prep homolog UNC-62 and the Exd/Pbx homologs CEH-20 and CEH-40 in C. elegans embryogenesis. Development. 2002;129:5255–68. doi: 10.1242/dev.129.22.5255. [DOI] [PubMed] [Google Scholar]
- 40.Spieth J, Lawson D. Overview of gene structure. WormBook. 2006;•••:1–10. doi: 10.1895/wormbook.1.65.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zahler AM. Pre-mRNA splicing and its regulation in Caenorhabditis elegans In: The C. elegans Research Community, ed. WormBook - Molecular biology -: http://www.wormbook.org, 2012:1-21. [DOI] [PMC free article] [PubMed]
- 42.Kuroyanagi H. Fox-1 family of RNA-binding proteins. Cell Mol Life Sci. 2009;66:3895–907. doi: 10.1007/s00018-009-0120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuroyanagi H, Watanabe Y, Suzuki Y, Hagiwara M. Position-dependent and neuron-specific splicing regulation by the CELF family RNA-binding protein UNC-75 in Caenorhabditis elegans. Nucleic Acids Res. 2013;41:4015–25. doi: 10.1093/nar/gkt097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hollins C, Zorio DA, MacMorris M, Blumenthal T. U2AF binding selects for the high conservation of the C. elegans 3′ splice site. RNA. 2005;11:248–53. doi: 10.1261/rna.7221605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sheth N, Roca X, Hastings ML, Roeder T, Krainer AR, Sachidanandam R. Comprehensive splice-site analysis using comparative genomics. Nucleic Acids Res. 2006;34:3955–67. doi: 10.1093/nar/gkl556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farrer T, Roller AB, Kent WJ, Zahler AM. Analysis of the role of Caenorhabditis elegans GC-AG introns in regulated splicing. Nucleic Acids Res. 2002;30:3360–7. doi: 10.1093/nar/gkf465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kabat JL, Barberan-Soler S, McKenna P, Clawson H, Farrer T, Zahler AM. Intronic alternative splicing regulators identified by comparative genomics in nematodes. PLoS Comput Biol. 2006;2:e86. doi: 10.1371/journal.pcbi.0020086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ast G. How did alternative splicing evolve? Nat Rev Genet. 2004;5:773–82. doi: 10.1038/nrg1451. [DOI] [PubMed] [Google Scholar]
- 49.Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–90. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hesselson D, Kimble J. Growth control by EGF repeats of the C. elegans Fibulin-1C isoform. J Cell Biol. 2006;175:217–23. doi: 10.1083/jcb.200608061. [DOI] [PMC free article] [PubMed] [Google Scholar]