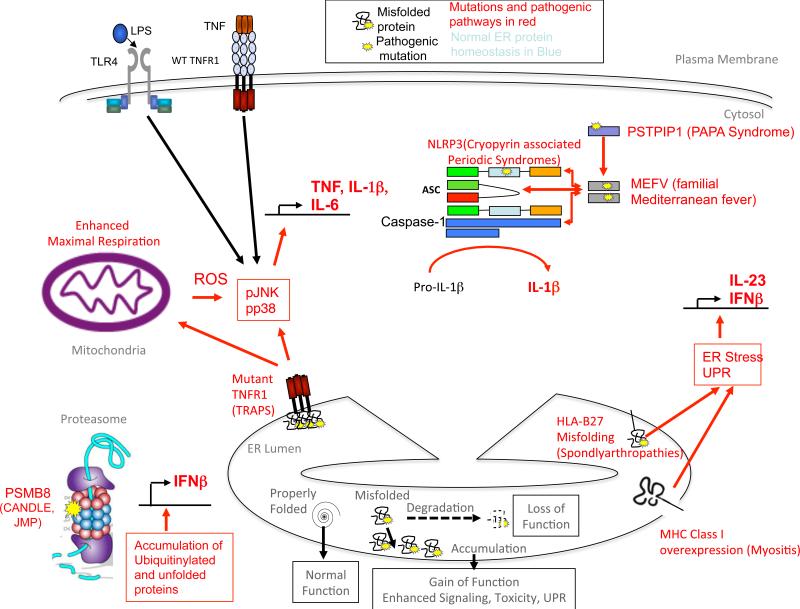
Figure 2. Consequences of protein misfolding and intracellular signaling complexes that activate autoinflammatory disease.
A) The outcomes of misfolding of secretory protewins in the ER are depicted at the bottom of the figure. Degradation of misfolded protein can cause a loss of function, whereas accumulation of misfolded proteins can trigger abnormal intracellular signaling, or at higher levels, induction of the unfolded protein response (UPR), which can also lead to the induction of inflammation and programmed cell death. Different foci of abnormal cellular signaling that trigger autoinflammatory diseases are depicted in the cell, clockwise from lower left. B) In PSMB8 deficiency, reduced degradation of misfolded proteins and peptides by the immunoproteasome leads to accumulation of ubiquinated proteins and cellular stress, which leads of Interferon-β production. Type I Interferon in turn upregulates the synthesis of immunoproteasome sububits, perpetuating the abnormalities. C) In TRAPS, extracellular mutations in TNFR1 lead to accumulation of the mutant receptor in the ER, which triggers an abnormal inflammatory response that is amplified by TNF or LPS signaling through cell surface receptors. D) In the Cryopyrin-Associated Perodic Syndromes (CAPS), mutations in NLRP3 enhance activation of the NLRP3 inflammasome and processing of IL-1β into its active form. E) In the spondylarthropathies, high-level expression of HLA-B27, which is enhanced in inflammation, fails to fold properly and is retained in the ER, triggering a partial ER stress response which leads to type I interferon and IL-23 production.