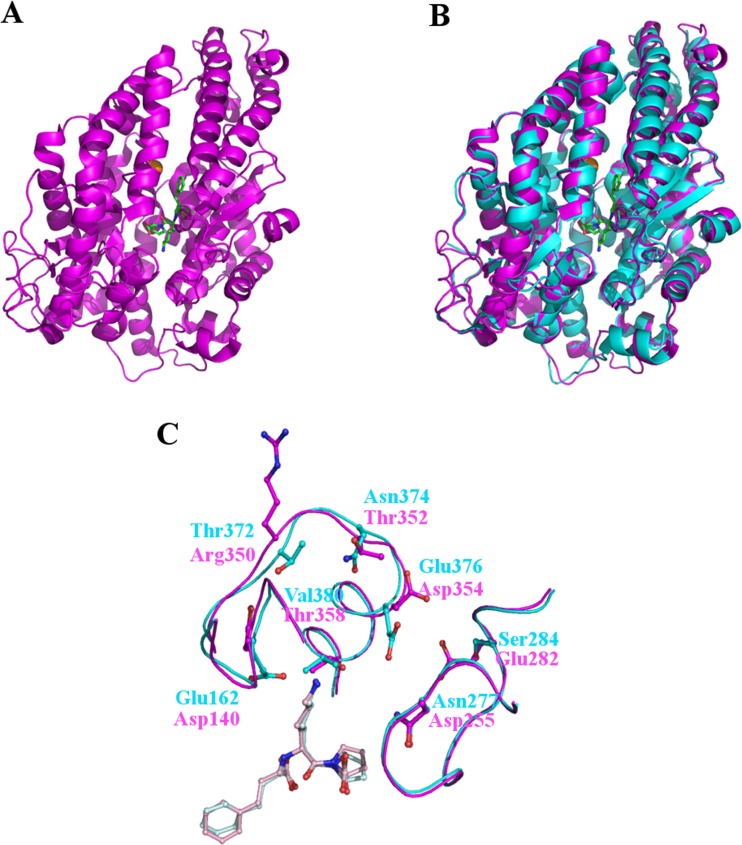
Fig. 3.
The structure of the N-domain of human sACE. (a) Cartoon representation of the overall N-domain structure (magenta) and (b) superposed on the structure of human tACE (cyan). The overall fold is identical save for an additional region at the C-terminus of the N-domain structure which serves as a linker region between the two domains. At the centre of the substrate binding channel the inhibitor lisinopril is visible as well as a zinc ion (grey sphere) and a single chloride ion (orange sphere). (c) Lisinopril binding to tACE and the N-domain. Although all of the residues forming direct interactions with Lisinopril are conserved between tACE and the N-domain, some differences are seen in the subsites particularly in the S1’ binding pocket. The subsite residues are show as cyan (tACE) and magenta (N-domain) sticks and labelled following the same colour scheme. The lisinopril bound to tACE is shown in light blue and from the N-domain in light pink. Important differences include the orientation of Glu162 in tACE compared to Asp140 in the N-domain which prevent interactions with the lysyl side chain of lisinopril in the N-domain at this position, and the position of the backbone at Thr372/Arg350 which prevents the long, positive side chain of arginine coming into contact with and repelling the lysyl side chain of lisinopril