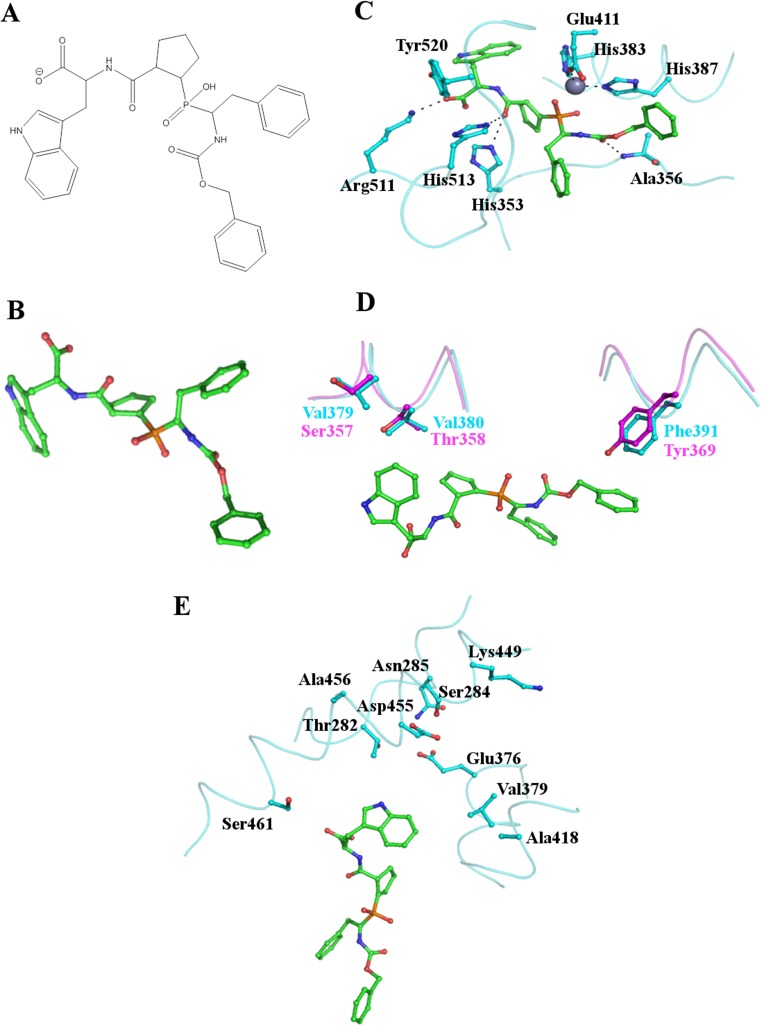
Fig. 4.
Binding of the C-domain specific inhibitor RXPA380 to tACE. (a) and (b) the structure of the C-domain specific inhibitor RXPA380. (c) Key residues of the tACE active site interacting with RXPA380. RXPA380 is shown as sticks with carbon atoms in green, the side chains of tACE residues are also shown as sticks with cyan carbon atoms. The active site zinc ion is shown as a grey sphere. RXPA380 binds in a similar conformation to lisinopril (Fig. 3). (d) Two key differences between tACE (cyan) and the N-domain (magenta) likely to decrease the affinity of RXPA380 for the N-domain. The Phe391/Tyr369 substitution eliminates the interaction with the phenyl moiety of the inhibitor whilst the Val379/Ser357 and Val380/Thr358 substitutions destroy the hydrophobic environment around the tryptophan group. (e) The S2’ binding pocket with side chains in close proximity to RXPA380 shown as sticks illustrates that the inhibitor does not fill this subsite and interactions here could potentially be increased to improve the efficacy of RXPA380