Abstract
Hoxb5 and Hoxa5 transcription factor proteins uniquely impact lung morphogenesis at the developmental time point when extremely preterm infants are born. The effect of O2 exposure (0.4 FiO2) used in preterm infant care on these Hox proteins is unknown. We used ex vivo fetal mouse lung organ cultures to explore the effects of 0.4 FiO2 on lung airway and vascular formation in the context of Hoxb5 and Hoxa5 expression and regulation. Compared to room air, 48 h (h) 0.4 FiO2 adversely attenuated airway and microvasculature formation while reducing lung growth and epithelial cell volume, and increasing mesenchymal volume. 0.4 FiO2 decreased pro-angiogenic Hoxb5 and VEGFR2 while not altering protein levels of angiostatic Hoxa5. Lungs returned to RA after 24 h 0.4FiO2 had partial structural recovery but remained smaller and less developed. Mesenchymal cell apoptosis increased and proliferation decreased with time in O2 while epithelial cell proliferation significantly increased. Hoxb5 overexpression led to prominent peri-airway VEGFR2 expression and promoted lung vascular and airway patterning. Hoxa5 overexpression had the opposite effects. We conclude that 0.4 FiO2 exposure causes a profound loss of airway and lung microvascular development that occurs partially via reduction in pro-angiogenic Hoxb5 while angiostatic Hoxa5 expression is maintained.
Keywords: Hoxb5, Hoxa5, Lung airway branching, Lung morphogenesis, Lung vasculogenesis
Introduction
Extremely preterm infants (23–24 weeks gestation) have a high degree of morbidity and mortality that is significantly associated with pulmonary complications from altered airway and vascular development. It is important to note that these most preterm infants are born when their lungs are transitioning from the late canalicular to the very early saccular stage of lung development. This is the earliest point when the air-vascular communication is being established. This is a critical period in development as the continuation of further airway arborization is essential to prepare for the subsequent formation of adequate alveolar structure and alveoli numbers that are needed to sustain adequate gas exchange (Inselman and Mellins 1981; Ten Have-Opbroek 1991; Jobe 2006). Significantly, important developmental stage-specific regulatory mechanisms that occur in the ex utero environment during this period of lung development are not addressed by most current research models, as these models focus on more structurally mature lungs during the saccular stage and the transition from the saccular to the alveolar stage (Laptook et al. 2005; Doyle et al. 2006; Higgins et al. 2007); (O’reilly 2001; Maniscalco et al. 2005; Barker et al. 2006; Aski et al. 2009). Additionally, while the deleterious effects of high O2 (>0.8 FiO2) are well known, very few studies have evaluated the effect of 0.4 FiO2 on the molecular control of lung development. (Maniscalco et al. 2005; Alejandre-Alcazar et al. 2006; Bustani et al. 2006; Bland et al. 2007).
Whole animal models also cannot readily address the mechanisms of lung injury during this critical period of late canalicular stage lung development. Thus, whole organ-based models are necessary to identify the specific molecular controls impaired by O2 exposure at this important time point in the developing lung. Such studies can then relate molecular regulation to structural development. This understanding may prove useful for devising new strategies that will target key regulatory pathways to allow for normal progression of lung development after extremely preterm birth.
Our work and that of others have shown the unique importance of the transcription factor proteins Hoxb5 and Hoxa5 in mouse and human lung particularly during the late canalicular to very early saccular stage of development. This developmental period is significantly relevant to preterm infants born at 23–24 weeks gestation. These studies indicate that Hoxb5 and Hoxa5 impact airway and alveolar development through cell-cell communication between the mesenchyme and epithelial cell compartments, but the exact mechanisms by which this occurs is not yet completely understood. (Aubin et al. 1997; Volpe et al. 1997, 2000, Golpon et al. 2001; Volpe et al. 2003; Kinkead et al. 2004; Mandelville et al. 2006; Volpe et al. 2007, 2008). An essential role played by mesenchyme to epithelial cell communication within the developing lung is the control of vessel formation within the lung mesenchyme that drives airway and alveolar development. (Hislop 2005; Stenmark and Abman 2005) In mesenchyme-derived cells from other tissues, Hoxb5 and Hoxa5 have critical but different roles in vascular cell fate, and angiogenesis (Wu et al. 2003; Rhoads et al. 2005; Winnik et al. 2009). Hoxb5 promotes endothelial cell differentiation from endothelial progenitor cells through regulation of VEGFR2 (Winnik et al. 2009). On the other hand, Hoxa5 has angiostatic or possibly antiangiogenic effects (Rhoads et al. 2005).
Despite these roles of Hoxb5 and Hoxa5 in control of vascular formation, nothing is known about Hoxb5 and Hoxa5 control of lung vascular development or how these roles of Hoxb5 and Hoxa5 impact airway morphogenesis. There is also no information on how hyperoxia alters Hoxb5 and Hoxa5 expression and regulation in developing lung. Uncovering these mechanisms is important because previous studies show that oxygen can affect methylation patterns and expression of regulatory factors, such as AP1 and NfkappaB that also control Hox transcription factor protein expression and activity.,(Shiraishi et al. 2002; Hershko et al. 2003) This effect of O2 on Hoxb5 and Hoxa5 regulation may be more important particularly at O2 levels of ≤ 0.4 FiO2 that are just as likely to alter genetic control even though less likely to cause cell death (Barker et al. 2006). Evaluating the 0.4 FiO2 level of O2 on molecular control with the developing lung is essential as this degree of O2 exposure is thought to be clinically safer than higher levels of O2 but the impact on developing lung is not completely understood (Aski et al. 2009).
Therefore our study was designed to explore the effects of modest hyperoxia, defined as 0.4 FiO2 exposure, on ex utero airway and vascular morphogenesis in the context of Hoxb5 and Hoxa5 expression patterns using an ex vivo whole lung model that encompasses the late canalicular stage of development. We hypothesized that modest O2 differentially regulates Hoxb5 and Hoxa5 expression patterns, leading to dysregulated airway and vascular morphogenesis.
Materials and methods
Animals
Timed-pregnant Swiss Webster mice at embryonic day (E) 14 (E0, morning of vaginal plug) were obtained from Charles River (Wilmington, MA). The animal study protocol was approved by the Institutional Animal Research Committee. Principles of laboratory animal care (National Institutes of Health publication 86-23, revised 1985) were followed.
Reagents
Antibodies: Hoxb5 rabbit polyclonal antibody was produced and characterized in our laboratory (Volpe et al. 2000). E-Cadherin rat monoclonal antibody was purchased from Invitrogen,(Grand Island, NY). Fluorescein conjugated lectin (GSL-B4) specific to endothelial cells was from Vector (Burlingame, CA). VEGF receptor 2 (VEGFR2) rabbit monoclonal antibody was from Cell Signaling (Danvers, MA) (Meyer et al. 1999). Rabbit polyclonal antibodies Hoxa5, ki67, and active caspase 3 was purchased from Abcam (Cambridge, MA) (Skalli et al. 1986; McGrath-Morrow et al. 2008). GAPDH mouse monoclonal was from Ambion (Austin, TX). Western blot horseradish peroxidase (HRP)-linked secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Immunostaining reagents were from Vector (Burlingame, CA); chemiluminescent reagent from Perkin Elmer (Boston, MA). The oxygen chamber was purchased from Billups-Rothenberg (Delmar, CA). All other reagents were from Fisher Scientific (Pittsburgh, PA) unless otherwise specified.
Whole fetal mouse lung organ cultures
Whole fetal mouse lung cultures were prepared as we have described with modifications (Volpe et al. 2000, 2007). In order to study the whole lung in organ culture, E14 fetal mouse lungs were cultured at the air-liquid interface for 48 h (h) in a 0.21 FiO2 (Room Air, RA), 5 % CO2, 37 °C incubator to allow the intact whole lungs to progress structurally to a developmental equivalent of late canalicular stage lung, as we and others have described (Slavkin et al. 1989; Volpe et al. 2000; Dieperink et al. 2006; Volpe et al. 2007; Bhaskaran et al. 2009). At 48 h, lungs were randomly assigned to the following conditions for an additional 48 h: 1) RA (Control Group); 2) 0.4 FiO2 (O2 Group) or; 3) 0.4 FiO2 for 24 h followed by RA for 24 h (0.4 FiO2 24 h/RA 24 h; Rescue Group). Lungs in these groups were cultured for total of 96 h (48 h in RA before treatment condition, then 48 h within the treatment condition) in the same incubator and monitored daily to confirm consistent O2 concentrations (by calibrated O2 meter). In a further set of experiments, lungs were transfected with Hoxb5 or Hoxa5 DNA expression plasmids or empty vector control using Dharmafect transfection reagent (Lafayette, CO) according to the manufacturer’s recommendations and as we have described (Yamaguchi et al. 1993; Volpe et al. 2007). Briefly, the expression plasmids were complexed with Dharmafect transfection reagent in serum-free medium followed by direct application of the plasmid-vehicle mixture (2 ul) to each lung that was rapidly absorbed by each lung. The remainder of the plasmid-vehicle mixture was mixed with growth medium in each culture dish. Using these methods we have previously shown effective uptake of constructs into lung tissue with specific changes in gene regulation without side effects (Volpe et al. 2007; Mujahid et al. 2013) The expression plasmids contain the complete open reading frame clone for Hoxb5 (Origene, Rockville, MD; Plasmid RG202156) or Hoxa5 (Origene, Rockville, MD; Plasmid RG218740). These plasmid transfected lungs were cultured for a total of 48 h. Nine separate experiments were performed each containing whole fetal lungs from 5 litters.
Evaluation of lung branching morphogenesis and lung morphologic growth
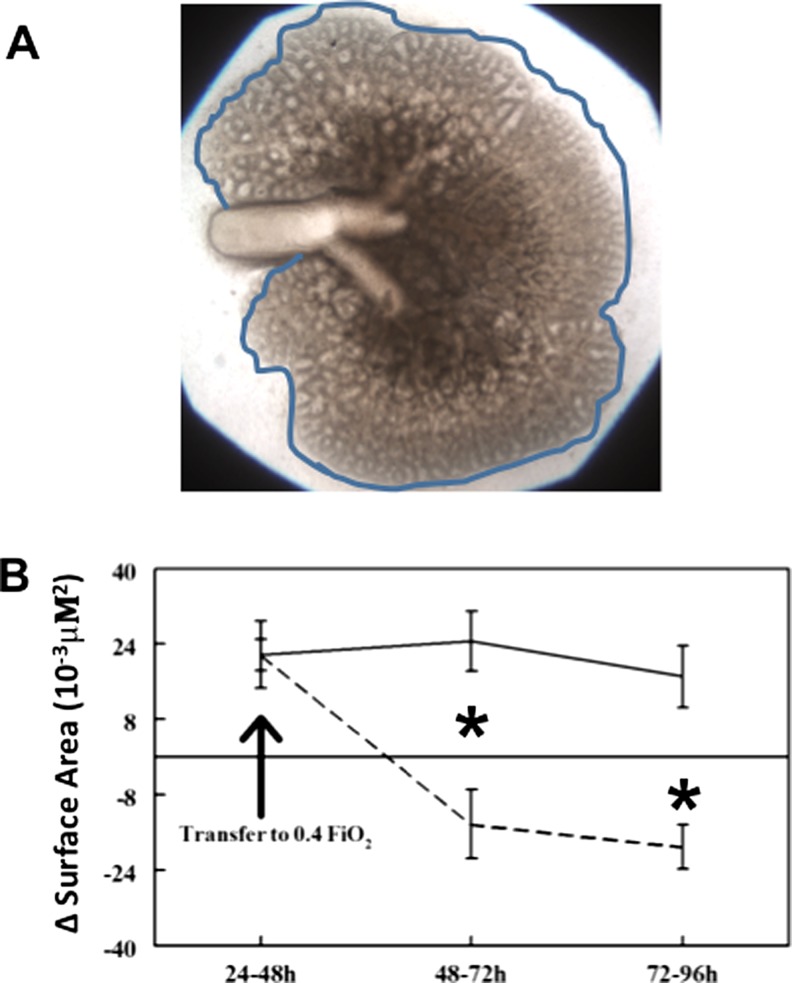
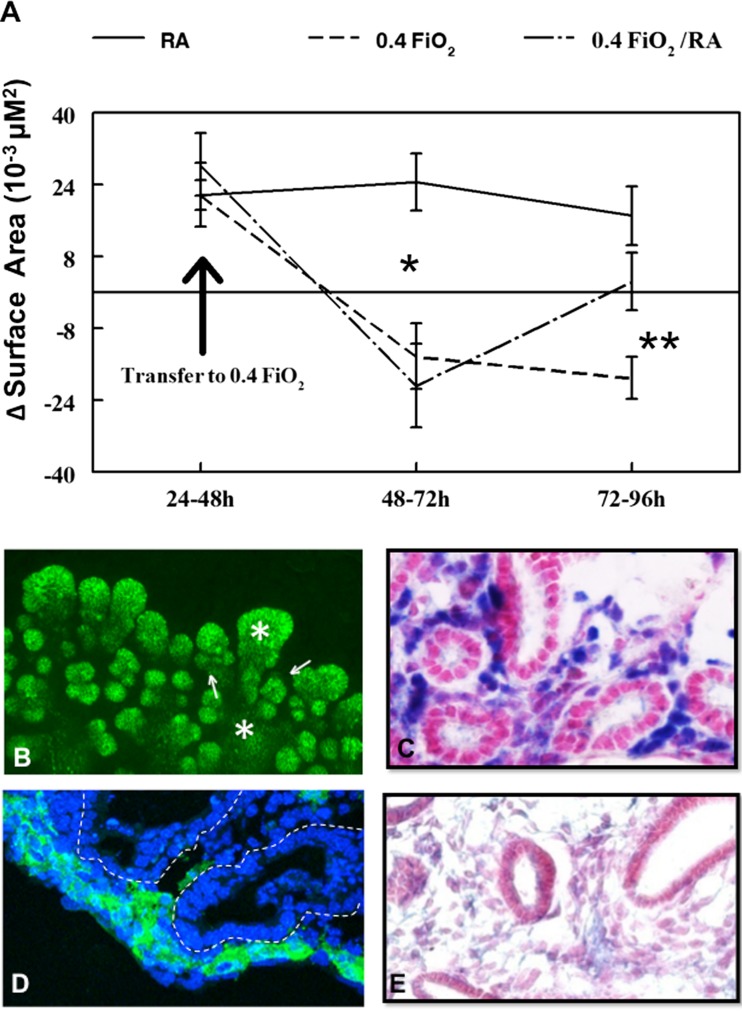
Lungs were microscopically visualized to compare daily growth and airway branching of each lung over time in culture. To quantify these visual evaluations, surface area evaluation was performed as we have previously done with modifications as follows (Volpe et al. 2000, 2003). Lungs confirmed at the initiation of culture to be whole lungs (all five lobes intact and identified) were photographed daily at 10x magnification. A computer-generated line (See Fig. 2a) was used to trace the outline of each whole lung followed by calculation of surface area within each outline. The generated surface area measurement of each lung was statistically compared to the surface area of same lung from the previous day of culture (∆SA). This allowed us to evaluate morphologic growth for each lung over time in culture in control (RA) versus experimental conditions (O2 and Rescue Group).
Fig. 2.
Lung morphologic growth identified by surface area (∆SA) analysis was adversely affected by 48 h 0.4 FiO2. a Example of whole lung growing in culture outlined by representative computer generated outline, generated for each lung and used to quantify lung surface area (Volpe et al. 2007). Area within outline is identified as lung surface area. b Change in surface area (∆SA) of lungs over time in culture: ∆SA is defined as surface area of each lung compared to itself from previous day of culture (i.e. lung surface area at 72 h subtracted by the same lung’s surface area at 48 h = ∆SA of that lung from 48 to 72 h of culture). Lungs that remained in RA (solid line) for 96 h had consistent growth as indicated by the positive ∆SA. However, lungs placed in 0.4 FiO2 after 48 h of culture (dashed line) had significantly regressed growth as indicated by the negative ∆SA. *P = 0.01, 0.4 FiO2 vs. RA at 48–72 h and 72–96 h, Mean ± SEM, N ≥ 20
E-cadherin and endothelial cell lectin immunofluorescence
Whole mount E-Cadherin (Invitrogen, Grand Island, NY) staining was done to identify how 0.4 FiO2 and induced expression of Hoxb5 and Hoxa5 impacted airway development (Lazarus et al. 2013; Mujahid et al. 2013). Lung sections were stained using a fluorescein-labeled GSL-B4 endothelial cell-specific lectin to assess vascular formation (Vector, Burlingame, CA). Using confocal microscopy, E-Cadherin- and endothelial cell lectin-labeled whole fetal lungs were visualized with image stacking along the z-axis. The generated Z-stacked images were used to visually assess whole lung structural airway branching and vessel formation. N ≥ 3 lungs per condition.
Western blot analysis
Western blot with densitometry was done as we have described to determine Hoxa5 and Hoxb5 total protein levels in lungs from the RA and O2 Groups and in lungs transfected with Hoxb5 and Hoxa5 DNA expression plasmids (Volpe et al. 2000, 2007). N = 8 samples/per condition with each sample containing 3 lungs.
Immunohistochemistry
Lung coronal tissue sections (N ≥ 3 lungs per condition from ≥ 3 experiments) were immunostained using Avidin-Biotin (ABC) methodology (Vector, Burlingame, CA) with blue alkaline phosphatase detection as we have described (Volpe et al. 2000, 2007, 2008). Primary antibody concentrations were: Hoxb5 (1/200); Hoxa5 (1/400); VEGFR2 (1/250); Caspase 3 (1/800) and ki67 (1/2000). All comparisons were made from reactions processed together with same chromagen exposure times. Immunostaining controls were done as we have published (Volpe et al. 2000, 2007). N ≥ 3 lungs per condition for each protein studied.
Lung morphometry (point counts)
Lung morphometry was measured to quantify phenotypic changes in lung morphology as we have previously described [29]. Using a computer generated 25 μM grid overlayed on a 20x magnification of selected tissue sections (separated by at least 18 μM), airway space, mesenchyme, and epithelial regions were identified at each intersecting point on the grid (point counts). The percentage mean of summed point counts of airway space, mesenchyme and epithelial cells from each tissue section were compared between Control and Experimental groups (N ≥ 30 sections per condition separated by 18 μm from ≥ 3 lungs lungs per condition).
Quantification of proliferating cells
Ki67+ epithelial and mesenchymal cells were counted in tissue sections from similar lung regions. The number of Ki67+ epithelial and mesenchymal cells was expressed as a percentage of Ki67+ cells within each tissue section with comparisons made between control and experimental groups. (N = ≥ 2 lungs per condition, at least three sections per lung each separated by at least 18 microns) (Bhatt et al. 2001).
Statistical analyses
Nonparametric ANOVA or two-tailed t-tests (GraphPad Software, San Diego, CA) were done as appropriate with significance level of P < 0.05.
Results
0.4 FiO2 altered lung morphologic development
Images of lungs were taken during culture. Whole lung confocal immunofluorescence of E-Cadherin (which labels lung epithelial cells) was done at the end of the culture period (Fig. 1). Fetal lungs appeared structurally similar at the start of culture and prior to the random assignment to Control (RA) and O2 (0.4 FiO2) treatment groups after 48 h of culture (Fig. 1a, a′ c, c′). Control lungs that remained in RA for the subsequent 48 h continued to form more branch generations from parent branches, developing a highly arborized airway pattern (Fig. 1b, b′). E-Cadherin immunofluorescence (Fig. 1e) further demonstrated the three-dimensional nature of airway branching in these control lungs. However, lungs switched to 0.4 FiO2 (Fig. 1d, d′, f) for the last 48 h of culture became noticeably less well arborized with arrested branching and apparent branch elongation of existing branches. Z-stacked E-Cadherin confocal images of the 0.4 FiO2 exposed lungs (Fig. 1f) confirmed that these oxygen-exposed lungs had a paucity of airway branches compared to control (RA exposed) lungs. Branches that were present appeared disorganized and wider than airways in control lungs. To further evaluate the visual impression of altered morphology with 0.4 FiO2 exposure we calculated the ∆SA (Fig. 2a), a reliable and quantifiable measure of lung growth in culture as we have previously done (Volpe et al. 2007). Over the first 48 h of culture while in RA, ∆SA was similar between groups. Control lungs grew at a consistent rate from 48 through 96 h of culture (∆SA at 48–72 h of 24 × 10−3 μM2 ± 6.4 and at 72–96 h of 20 × 10−3 μM2 ± 4.7, mean ± SEM; Fig. 2b). In contrast, within 24 h of 0.4 FiO2 exposure, ∆SA was significantly decreased compared to controls (∆SA at 48–72 h of −14.2 × 10−3μM2 ± 7.3, Fig. 2b; P = 0.01) and further regressed in the next 24 h (∆SA at 72–96 h of −19 ± 4.7 μM2, mean ± SEM; Fig. 2b; P = 0.01).
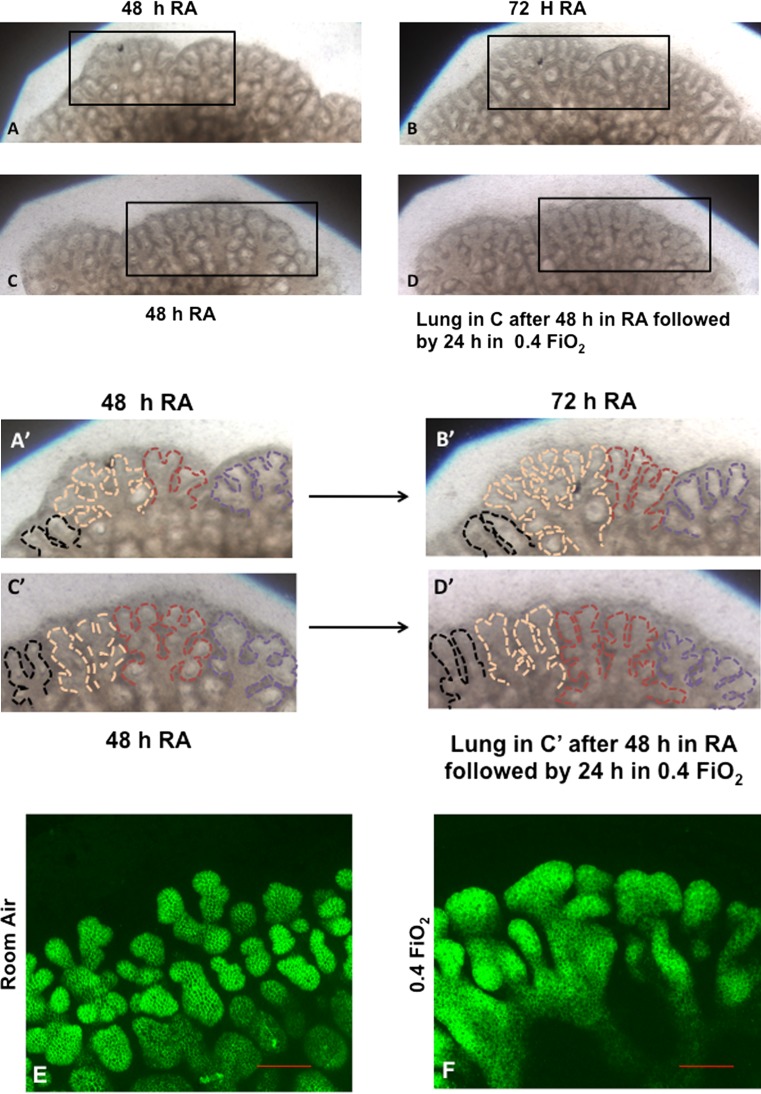
Fig. 1.
0.4 FiO2 exposure alters airway branching in ex vivo fetal mouse lungs. a–d: Light microscopy images of representative regions of whole fetal mouse lungs growing in room air or 0.4 FiO2 (10×). a, c view of the same region of two different whole lungs cultured for 48 h in RA. b Same area of whole lung seen in ‘a’ after 72 h in RA. d Same area of lung seen in ‘c’ 24 h after being switched to 0.4 FiO2. Squares in a–d outline areas of higher magnification shown in a′–d′ where epithelial boundaries are outlined by dashed lines to better illustrate airway branching patterns. Lungs that remained in RA (a, a′, b, b′) continued to show the development of more branch generations whereas the lung switched to O2 (d, d′) showed an arrest of airway arborization. (N ≥ 20 lungs per condition) e, f: E-Cadherin immunofluorescence confocal images of whole fetal lungs at end of culture period. Similar lung regions from whole mount images taken within the same plane are shown for lungs cultured in RA (e) or O2 (f) to further demonstrate the comparison between airway branching in each group (Bar is 100 μ, 20×). The lung in ‘e’ remained in RA culture for entire 96 h, whereas the lung in ‘f’ was switched to 0.4 FiO2 for 48–96 h of culture (48 h O2 exposure). Lungs remaining in RA for the entire culture period developed a three dimensional network of airway branches (e). In comparison, lungs that were switched to 0.4 FiO2 (f) had a paucity of airway arborization. Airways that were present were grossly dilated and had less well defined E-Cadherin staining suggesting a loss of epithelial cell organization or adhesion. (N = 2−3 lungs per condition)
Impact of modest O2 exposure on the developmental expression patterns of Hoxb5 and Hoxa5 transcription factor proteins
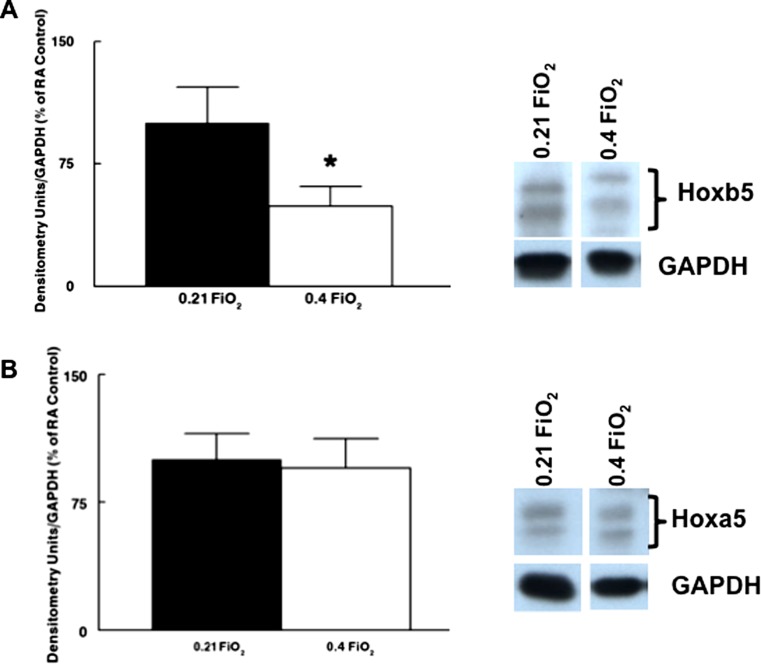
Knowing the unique importance of Hoxb5 and Hoxa5 to lung development compared to other Hox proteins expressed in developing lung (Bogue et al. 1994; Aubin et al. 1997; Volpe et al. 1997, 2000, 2003), we evaluated Hoxb5 and Hoxa5 protein levels and immunolocalization in lungs from the RA and O2 groups. After 48 h of 0.4 FiO2, Hoxb5 protein levels decreased to 50% of control lungs (Fig. 3a; P = 0.0009). Hoxa5 total protein levels were not significantly changed (Fig. 3b). This change in Hoxb5 while Hoxa5 protein levels were relatively unchanged altered the normal developmental expression pattern of these two Hox proteins, towards that observed later in lung development (near term gestation) when airway arborization is completed and saccularization is well-established (Volpe et al. 1997, 2003, 2008). The significant change in the levels of Hoxb5 without any substantial change in Hoxa5 with 0.4 FiO2 exposure is further reflected by the altered spatial and cellular distribution of Hoxb5 and Hoxa5 (Fig. 4). In RA-cultured lungs, Hoxb5 nuclear staining (Fig. 4a) was localized more to fibroblasts around distal airway branches than to regions around central lung airways. Hoxa5 (Fig. 4c) was localized diffusely throughout the mesenchyme. These expression patterns are similar to what we have shown in in vivo developing lungs at approximately E17-E18 mouse and 22–23 wk human lung (Volpe et al. 1997, 2003, 2008). In contrast, the airways of lungs cultured in 0.4 FiO2 for 48 h (Fig. 4b, d) exhibited a predominance of columnar epithelium characteristic of early canalicular stage lungs. The normal pattern of Hoxb5 spatial and cellular expression was lost. Mesenchymal Hoxb5 expression was much less intense and showed no obvious spatial distribution (Fig. 4b). In contrast, Hoxa5 protein became relocalized to anomalous clusters of condensed mesenchymal cells (Fig. 4d).
Fig. 3.
Effect of 0.4 FiO2 on Hoxb5 and Hoxa5 protein levels (Western blot and densitometry) in ex vivo fetal mouse lungs. Lungs with O2-induced altered branching had significantly decreased Hoxb5 (a) but unchanged Hoxa5 (b) protein levels. *P = 0.0009, Mean ± SEM, N = 8
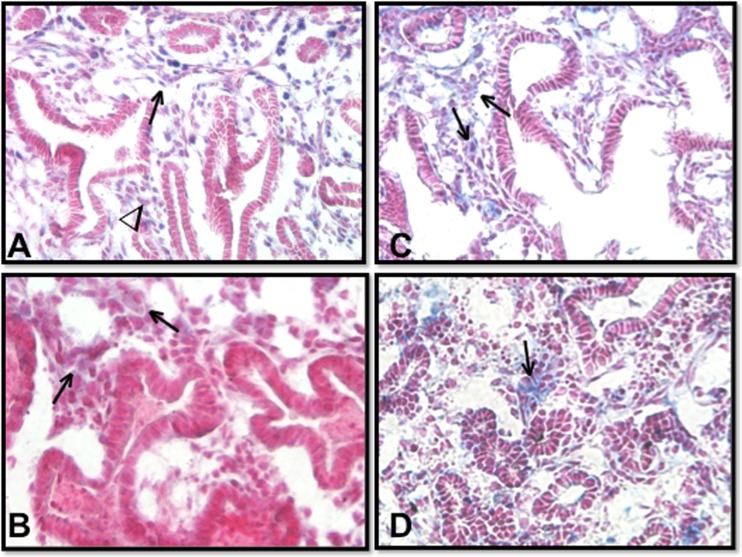
Fig. 4.
0.4 FiO2 profoundly changed Hoxb5’s expression pattern and altered mesenchymal localization of Hoxa5. Lung periphery is at top and central lung at bottom of each picture (40× Mag). RA lungs (a, c) had many variable-sized airways lined by columnar and cuboidal epithelium: Hoxb5 protein (blue nuclear staining) localized to mesenchyme around peripheral airways (→in a) more than around central airways (∇ in a). O2 exposed lungs (b, d) had narrower lumens with more columnar epithelium: Hoxb5 staining was less noticeable (→in B) with no obvious distribution. Compared to the more diffuse Hoxa5 staining in RA lungs (c), with O2 (d) Hoxa5 (blue staining) protein was mostly localized to cell clusters within the mesenchyme (⇒in d). N = 5 lungs/condition from 5 experiments
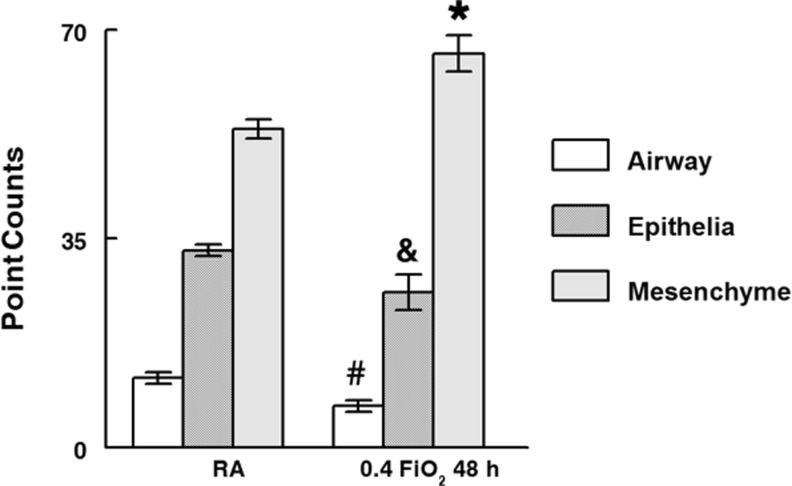
Morphometric analysis further delineated the gross morphologic changes seen in the cultured O2 exposed lungs (Fig. 5). Compared to RA control lungs, lungs placed in 0.4 FiO2 from 48 to 96 h had significantly decreased airway (P = 0.003) and epithelial cell volume (P = 0.002) and increased mesenchymal volume (P = 0.04).
Fig. 5.
Morphometry (point counts) quantified lung regions affected by 0.4 FiO2. Compared to RA, O2-exposure significantly decreased airway space (40 %↓) and epithelial cell volume (7 %↓) but increased mesenchyme volume (13 %↑). #P = 0.003; &P = 0.04;*P = 0.002, O2 versus RA, Mean ± SEM, N ≥ 30 tissue sections each separated by at least 18 μm from ≥ 3 lungs/condition
Modest O2-induced changes in endothelial cell fate correlated with altered Hoxb5 and Hoxa5 expression patterns
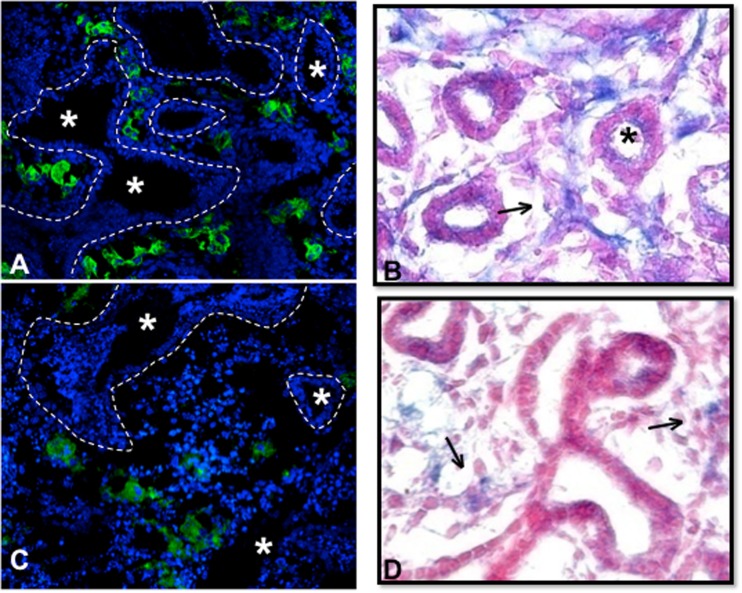
Knowing that airway development and lung vascular development can reciprocally affect each other (Coalson 2003; Thebaud et al. 2005) and that Hoxb5 and Hoxa5 have prominent but opposing roles in vascular development in other organs, (Wu et al. 2003; Rhoads et al. 2005; Winnik et al. 2009) we compared the changes in Hoxb5 and Hoxa5 expression patterns observed in our model with changes in lung endothelial cell organization (identified by endothelial cell-specific lectin binding) and VEGFR2 expression patterns. RA Control lungs showed the expected pattern of peri-airway localization of endothelial cells and VEGFR2 expression (Fig. 6a, b). With 0.4 FiO2 exposure, this peri-airway distribution (Fig. 6c, d) was abolished. Lectin binding was reduced and only seen in remotely isolated clusters of cells around apparent damaged and disorganized airway structures (Fig. 6c). Similarly, overall VEGFR2 staining was less intense and VEGFR2 positive cells within lung mesenchyme were more distant from airway epithelium in 0.4 FiO2 exposed lungs (Fig. 6d).
Fig. 6.
0.4 FiO2 altered lung vascular patterning and VEGFR2 protein localization. Lungs that remained in RA for the entire culture period (a, b) developed apparent vascular tufts (green Lectin IF in (a) and robust VEGFR protein localization (arrows in b) that surrounded developing airways (*). However, lungs that were exposed to 0.4 FiO2 for 48 h had decreased evidence of vessel formation (c) around airways (*) that had lost some of their structural integrity and had only isolated VEGFR2 positive cells (arrow in d). Epithelial boundaries are outlined by dashed lines in a and c to better identify airway structures in these immunofluorescent images. N ≥ 3 lungs/condition from at least 3 experiments
Hoxb5 and Hoxa5 directly impact lung airway and microvascular development
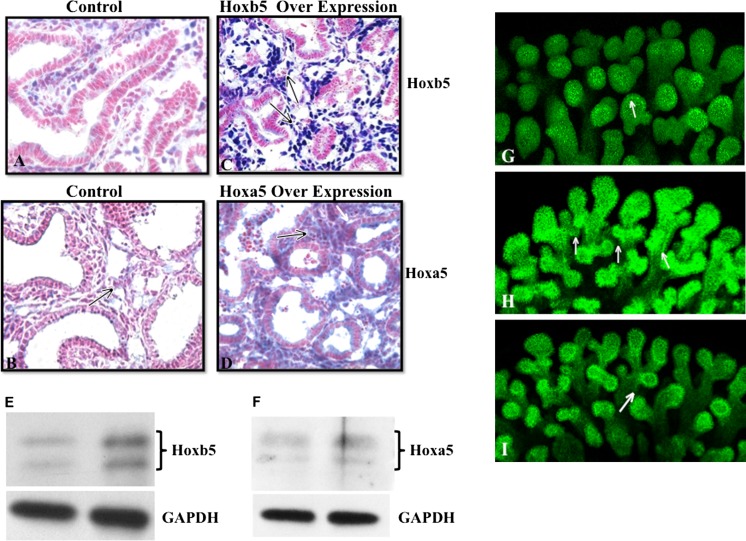
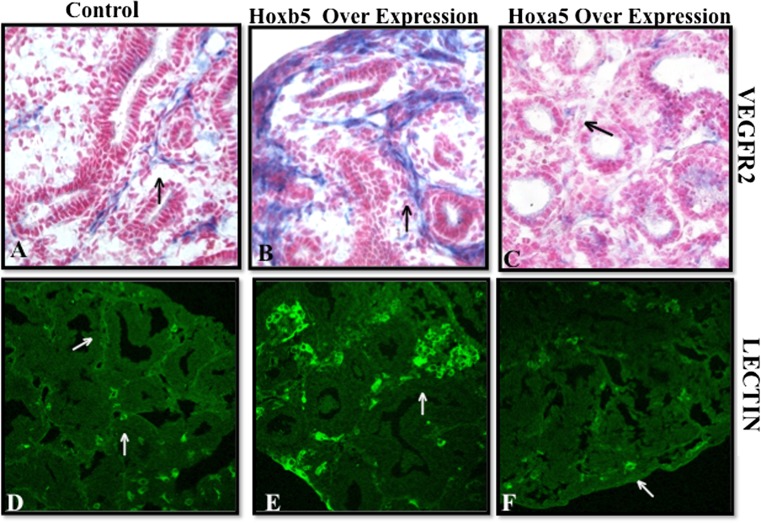
We further evaluated the direct effect of induced Hoxb5 and Hoxa5 expression on airway branching patterns (whole mount E-Cadherin staining), VEGFR2 localization and lung microvascular development (lectin binding). Compared to controls (Fig. 7a, b), lungs with induced Hoxb5 expression had much more intense peri-airway Hoxb5 mesenchymal cell localization (Fig. 7c) and increased Hoxb5 protein levels (Fig. 7e). Induction of Hoxa5 led to more diffuse Hoxa5 mesenchymal expression (Fig. 7d) and increased Hoxa5 protein levels (Fig. 7f). Hoxb5 and Hoxa5 induction caused unique changes in airway branching, VEGFR2 staining and endothelial cell organization compared to control lungs. Hoxb5 over expression led to the development of airway branches that had multiple branch points (Fig. 7h). Lungs with Hoxa5 over expression had a more finely arborized pattern (Fig. 7i). Evaluating VEGFR2, an early marker used to identify cells with an endothelial phenotype, we found that Hoxb5 over expression also caused an increase in peri-airway localization of VEGFR2 (Fig. 8b) and enhanced endothelial cell organization (Fig. 8e) suggesting the formation of robust vessel clusters. Hoxa5 over expression, on the other hand, reduced peri-airway VEGFR2 localization (Fig. 8c) and lectin binding identified isolated small clusters of endothelial cells within the lung and at the edge of the lung (Fig. 8f). No differences were seen in localization or amount of Ki67 expressing cells within the transfected lungs compared to control lungs (data not shown) suggesting that these effects of Hoxb5 and Hoxa5 on microvasculature and airway formation do not occur through a prominent change in cell proliferation. We also did not see any change in airway epithelial differentiation (Sox2 and TTF-1 immunostaining, data not shown) suggesting that the altered airway arborization involves a different mechanism from airway epithelial differentiation.
Fig. 7.
Over expression of Hoxb5 or Hoxa5 protein in fetal mouse lungs caused different changes in airway branching patterns. In control lungs (a, b), scattered Hoxb5 (a) and Hoxa5 (b) positive cell (blue staining) clusters were seen underlying the airway epithelium. However, Hoxb5 plasmid transfected lungs (c) had intense and diffuse staining (arrows in c) surrounding airways with narrow lumens and apparent multiple branch points. Hoxa5 transfected lungs (d) had diffuse mesenchyme expression of Hoxa5 (arrows in d) surrounding developing airways with wider lumens than the Hoxb5 transfected lungs. e, f Western blot confirms increased protein levels when lungs were transfected with either the Hoxb5 (e) or Hoxa5 (f) expressing plasmid. g, h, i E-Cadherin whole mount confocal images show that compared to control lungs (g), Hoxb5 transfected lungs (h) had multipodal and 3D airway structures (arrows in h). Hoxa5 transfected lungs (i) developed a more finely arborized airway branching pattern (arrows in i) that appeared better organized than that seen with Hoxb5 over expression. 20× Mag, N = 3 lungs per condition
Fig. 8.
Hoxb5 or Hoxa5 over expression caused specific changes in VEGFR2 protein localization (a–c) and lectin-labeled vascular endothelial cells (green IF in d–f). Compared to control lungs (a, d), Hoxb5 over expression led to more prominent peri-airway VEGFR2 localization (b, arrow) and increased presence of endothelial cells (e, arrow) suggesting development of robust vessel clusters. Hoxa5 over expression (c) limited the degree of peri-airway VEGFR2 and the vascular pattern surrounding these airways was more simplified suggestive of limited vessel aborization (f, arrow). Mag. 40× (a–c), 20× (d–f), N = 2
Return of lungs to RA after 24 h 0.4 FiO2 exposure (Rescue group) led to some recovery of lung growth and airway and vascular development in association with modified Hoxb5 cellular expression patterns
Figure 9a expands upon results shown in Fig. 2, adding to the data to show the comparison of surface area in lungs returned to RA after 24 h exposure to oxygen verses lungs in the Control (RA) or O2 (0.4 FiO2) group. Lungs designated as rescue group lungs where cultured similarly and had similar surface area to lungs cultured in O2 until they were returned to RA after 24 h of 0.4 FiO2 exposure. Lungs returned to RA after 24 h exposure to 0.4 FiO2 exhibited some recovery of lung growth (dashed-dot line, Fig. 9a) but they remained smaller than RA cultured lungs. Images of Z-stacked E-Cadherin- whole mount lung staining (Fig. 9b) demonstrated that this recovery of lung growth occurred with the formation of new airways on a background of the dilated or widened airways that were formed during 0.4 FiO2 exposure (compare Fig. 9b to Fig. 1d). These changes in airway branching correlated with some recovery of peri-airway localization of Hoxb5 (Fig. 9c), lectin binding (Fig. 9d), and VEGFR2 expression (Fig. 9e). Hoxa5 mesenchymal expression in the rescue group was intermediate compared to RA and 48 h O2 lungs (data not shown).
Fig. 9.
Rescue group lungs had partial recovery of lung growth and changes in Hoxb5, VEGFR2 and Lectin localization. a Compared to lungs that remained in O2 for 48 h (dashed lines), lungs that were returned to room air after 24 h 0.4 FiO2 exposure (Rescue group, dashed-dot line) had increased ∆SA. b E-Cadherin whole mount staining of Rescue lungs demonstrated formation of new airway buds (↑) from dilated airways (*). New peripheral airways were also surrounded by Hoxb5 positive mesenchymal cells (c), lectin (d, epithelial boundaries outlined by dashed lines) and VEGFR2 (e) around these airways suggesting some recovery of vessel formation. Mag 20× (b) and 40× (c–e), N = 3
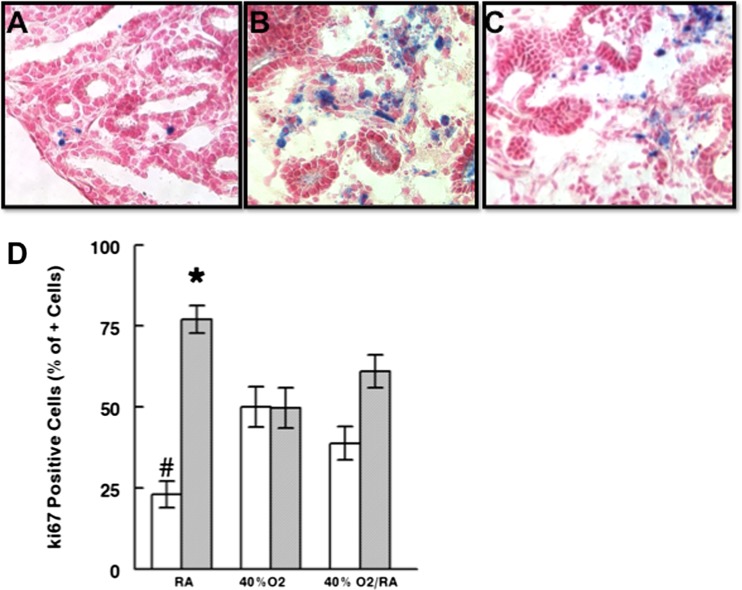
Modest O2 exposure changed active caspase 3 and Ki67 protein cellular localization
To understand the contribution of apoptosis and cellular proliferation to lung structural changes seen with ex utero exposure to this level of O2 we evaluated the cellular localization pattern of active caspase 3 and Ki67. Active caspase 3, an indicator of the degree of apoptosis, was rarely seen in control lungs (Fig. 10a) (Barker et al. 2006). After 0.40 FiO2 exposure (Fig. 10b), caspase 3 was preferentially localized to clusters of mesenchymal cells. Rescue lungs (Fig. 10c) had an intermediate degree of caspase-positive cells within the mesenchyme. Evaluation of the percentage of Ki67 positive cells (detects cycling cells) (Maniscalco et al. 2002; Bustani et al. 2006) demonstrated that compared to RA control lungs epithelial proliferation was significantly increased and mesenchymal cell proliferation significantly decreased with length of time in 0.40 FiO2 (Fig. 10d; P = 0.04).
Fig. 10.
Active caspase 3 and Ki67 cellular localization changed with length of exposure to 0.4 FiO2. Compared to RA (a), caspase 3 staining was more diffuse in mesenchyme of lungs exposed to 0.4 FiO2 for 48 h (b). Rescue lungs returned to RA after 24 h 0.4 FiO2 (c) had intermediate caspase 3 positivity. 40× Mag, N = 3 (d) Quantification of the percent of Ki67+ cells revealed significantly increased epithelial (white bars) and decreased mesenchymal (grey bars) cell proliferation with length of time in 0.4 FiO2. *, # P = 0.04, RA vs. 0.4 FiO2 and 0.4 Fi O2/RA (Rescue group), Mean ± SEM, N = 3−4 sections per lung from 2 to 3 lungs/condition
Discussion
Preterm infants born at the limits of viability (23–24 weeks) gestation have a severely compromised chance of survival (Tyson et al. 1999; Hamilton et al. 2009). They are born when their lungs are in transition from the late canalicular to very early saccular stage of structural development. During this time period, airway and vascular development has reached a point to potentially allow sufficient gas exchange in the extra-uterine environment but only with significant respiratory support including oxygen. In infants at this gestational age, any significant alteration in the lung’s structural developmental program will occur well before the onset of alveolar formation, placing them at the highest risk for short- and long-term pulmonary morbidity. To understand how lung development is curtailed in these extremely preterm infants, it is necessary to understand how their earlier birth and exposure to hyperoxia affects airway and vascular development. Understanding lung-specific mechanisms that occur at this time point in development will shed light on the precursor molecular events that ultimately lead to defective alveolar formation. This knowledge is essential to developing therapies that can promote continued normal airway and vascular development for infants born at the limits of viability. Animals rarely survive if born at this stage of prematurity, which precludes the evaluation of ex utero therapy on the relationship between structural development with specific protein expression in the intact animal. Therefore, we used an ex vivo whole lung experimental model. This allowed us to focus on alterations in the lung’s developmental program at a developmental time point more relevant to that of infants born at 23–24 weeks gestation whose lungs are then exposed to the extra- uterine environment. We chose to focus on exposure to relatively modest O2 levels (0.4FiO2) given the recently revised resuscitation guidelines for preterm infants and that the concentration of O2 at or around 0.4 FiO2 has traditionally been thought to be less toxic to lung than higher O2 concentrations (van Oostveen et al. 1999; Aski et al. 2009). Given the importance of Hoxb5 and Hoxa5 to the lung structural development and the distinct roles for these Hox proteins in vasculogenesis and angiogenesis (Wu et al. 2003; Rhoads et al. 2005; Arderiu et al. 2007; Winnik et al. 2009), we focused on these Hox proteins in our evaluation. We show that even modestly increased oxygen (0.4FiO2) significantly altered Hoxb5 expression changing the balance between lung mesenchyme expression of angiogenic Hoxb5 and angiostatic/anti-angiogenic Hoxa5, while adversely changing airway branching, lung morphology, and vasculogenesis. Further, we have shown for the first time the direct impact of regulation by these Hox proteins on lung blood vessel formation.
Our results suggest that 0.4 FiO2 decreases airway generations from which saccules and alveoli can form with progression of development. Our visual interpretation of lungs in culture and morphometric data showing a decrease in lung area composed of airway space compared to mesenchyme area with O2 exposure supports this possibility. The rapidity of arrest in airway development (within 24 h O2) and the lack of complete recovery over a similar time period when lungs are returned to room air indicates the potential for permanent effects of even short periods of 0.4 FiO2 exposure. While this model cannot reproduce and is not meant to be a perfect comparison to the clinical experience of infants born at 23–24 weeks gestation, it is an accessible and most feasible way to evaluate in structurally intact whole lungs the effects of ex utero oxygen exposure at the late canalicular to very early saccular stage transition. Further, it uncovers important aspects of lung development to consider that are still in play when infants are born at these extremes of prematurity with incomplete lung structure. This is also important information as it identifies structural alterations in ex utero lung morphogenesis that occur at a time in development that is prior to the onset of the alveolar phase. In human infants, these changes can only be studied retrospectively in post-mortem samples long after the initial injury to the developing lung.
In the current study, the mechanism causing reduced Hoxb5 expression with 0.4 FiO2 exposure is possibly a result of the ability of oxygen to regulate the expression and DNA binding of transcriptional complexes including AP1 and CEBP that have binding sites in the Hoxb5 promoter region (Galang and Hauser 1993; Rahman et al. 2006; Wright and Dennery 2009; Xu et al. 2009; www.cbil.upenn.edu/cgi-bin/tess/tess2010). Oxygen can also alter DNA methylation. (Barker et al. 2006) Hoxb5 expression is modulated via methylation which may have played a role in down regulation of Hoxb5 in our study (Hershko et al. 2003). We previously showed that specific inhibition of Hoxb5 protein expression in developing mouse lung led to arrested airway branching and aberrantly placed airway generations with changes in cell-cell adhesion at the mesenchyme-epithelial border of developing airways (Volpe et al. 2000, 2007). Conversely, in human congenital lung anomalies characterized by increased airway branching, Hoxb5 protein is abnormally up regulated is association with altered expression of cell-cell adhesion molecules (Volpe et al. 2003). These studies, together with the data we present here, strongly suggest that decreased Hoxb5 protein levels are part of the mechanism leading to the arrest and regression of airway development with 0.4 FiO2 ex utero exposure.
The preferential effect of 0.4 FiO2 exposure on decreasing Hoxb5 protein levels but not significantly impacting Hoxa5 protein levels is very noteworthy considering the opposing roles of Hoxb5 and Hoxa5 in vasculogenesis and angiogenesis in other tissues and in our current study (Wu et al. 2003; Rhoads et al. 2005; Winnik et al. 2009). In human umbilical vein endothelial cells, Hoxb5 promotes progression of endothelial cell fate and differentiation through direct up regulation of VEGFR2 expression via direct binding of Hoxb5 to the VEGFR2 promoter (Wu et al. 2003; Winnik et al. 2009). In this same cell type, Hoxa5 inhibits the expression of several proangiogenic substances including VEGFR2, decreasing vessel branching (Rhoads et al. 2005). Our data support that reduced mesenchymal expression of proangiogenic Hoxb5 while expression of angiostatic Hoxa5 remains relatively unchanged likely contributes to decreased peri-airway blood vessel formation in late canalicular stage lungs after 0.4 FiO2 exposure. Loss of peri-airway blood vessels would be expected to adversely impact airway development via changes in both cell-cell interactions and altered paracrine and juxacrine signaling (Hislop 2005; Stenmark and Abman 2005). Although Hoxa5 total protein levels did not significantly change, the locally altered Hoxa5 mesenchymal expression pattern and reduced Hoxb5 expression is likely important. Small changes in the cellular expression of a Hox protein can result in significant downstream changes in gene regulation (Graba et al. 1997; Hombria and Lovegrove 2003). The expression pattern of Hoxa5 in lungs exposed to 0.4 FiO2 in this study is similar to that seen in a mouse model of pulmonary hypoplasia (Volpe et al. 2008).
Oxygen can also alter cell proliferation and apoptosis, in part through altered regulation of specific proteins that control these cellular events (Barker et al. 2006; Wright and Dennery 2009). Hox proteins can either promote or inhibit cell proliferation depending on the cellular environment and presence or absence of Hox cofactors (Morgan et al. 2000; Pan and Simpson 2001). In this study we found that 0.4 FiO2 exposure to the ex utero lung altered mesenchymal cell apoptosis and airway and mesenchymal cell proliferation. This did not appear to occur through direct changes in Hoxb5 and Hoxa5 as direct over expression of Hoxb5 or Hoxa5 in lung organ cultures did not produce significant changes in either active caspase 3 or ki67 immunostaining (data not shown). However, mesenchymal cell apoptosis was increased and proliferation decreased in proportion to the length of time the lungs remained in 0.4 FiO2. This information along with the selective reduction of Hoxb5 in mesenchyme suggests that this increased mesenchyme apoptosis may have targeted cells expressing Hoxb5 that were possibly progenitor cells for future blood vessel formation (Wu et al. 2003; Winnik et al. 2009). This possibility is supported by other published studies showing the important role of Hoxb5 in the regulation of mesenchymal precursor cell development into mature endothelial cells and through the known direct regulatory role of Hoxb5 on VEGFR2 expression (Wu et al. 2003). VEGFR2 regulation is one of the factors that direct mesenchymal cells to an endothelial cell fate (Yamaguchi et al. 1993). On the other hand, continued mesenchymal expression of the more angiostatic Hoxa5 in the face of this decreased Hoxb5 expression with 0.4 FiO2 exposure may have favored a premature decrease in vessel branching around developing airways (Rhoads et al. 2005; Arderiu et al. 2007). The change in epithelial cell proliferation that we noted in our study is similar to findings of others where higher oxygen concentrations were used (Maniscalco et al. 2002; Bustani et al. 2006). This altered epithelial cell proliferation may be a compensatory response to the loss of airway arborization and could have occurred via direct effects on epithelial cell function or through altered mesenchymal-epithelial cell interactions (Stenmark and Abman 2005).
Conclusions
In summary, this work suggests that 0.4 FiO2 exposure in the late canalicular stage lung leads to abnormal airway and vascular morphogenesis partially through a decrease in Hoxb5 protein expression in lung mesenchyme. This decreased expression of proangiogenic Hoxb5 in the face of the continued presence of angiostatic or anti-angiogenic properties of Hoxa5 protein likely contributes to arrest of lung vascular formation at this time in development. In human infants, this altered developmental regulation may be part of the mechanism that sets the stage for the beginnings of impaired lung development after preterm birth and may partially explain the mechanisms contributing to the severe pulmonary morbidity and mortality of infants born at the limits of viability. Examining these mechanisms during this critical developmental window is important for advancing insight into gestational-age specific events that contribute to the continued poor pulmonary outcome in this group of infants.
Acknowledgments
Funding: HD044784, Tufts Medical Center Research Grant, AAP Klaus Award, Ikaria Research Award, HL037930, P30 NS047243, Peabody Foundation.
Abbreviations
- ELBW
Extremely low birth weight
- ∆SA
Change in surface area
- E
Embryonic day
- h
Hour
- RA
Room air
- VEGFR
Vascular endothelial growth factor receptor
Footnotes
Francheyska Silfa-Mazara and Sana Mujahid contributed equally.
References
- Alejandre-Alcazar MA, Kwapiszewska G, Reiss I, Amarie OV, Marsh LM, Sevilla-Perez J, Wygrecka M, Eul B, Kobrich S, Hesse M, Schermuly RT, Werner S, Eickelberg O, Morty RE. Hyperoxia modulates TGF-b/BMP signaling in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2006;292:L537–L549. doi: 10.1152/ajplung.00050.2006. [DOI] [PubMed] [Google Scholar]
- Arderiu G, Cuevas I, Chen A, Carrio M, East L, Boudreau NJ. HoxA5 stabilizes adherens junctions via increased Akt1. Cell Adh Migr. 2007;1(4):185–195. doi: 10.4161/cam.1.4.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aski LM, Henderson-Smart DJ, Ko H (2009) Restricted versus liberal oxygen exposure for preventing morbidity and mortality in preterm or low birth weight infants. Cochrane Database Syst Rev 1 [DOI] [PMC free article] [PubMed]
- Aubin J, Lemieuz M, Tremblay M, Berard J, Jennotte L. Early postnatal lethality in Hoxa-5 mutant mice is attributable to respiratory tract defects. Dev Biol. 1997;192:432–445. doi: 10.1006/dbio.1997.8746. [DOI] [PubMed] [Google Scholar]
- Barker GF, Manzo ND, Cotich KL, Shone RK, Waxman AB. DNA damage induced by hyperoxia. Am J Respir Cell Mol Biol. 2006;35:277–288. doi: 10.1165/rcmb.2005-0340OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskaran M, Wang Y, Zhang H, Went T, Baviskar P, Guo Y, Gou D, Liu L. MicroRNA-127 modulates fetal lung development. Physiol Genomics. 2009;37:268–278. doi: 10.1152/physiolgenomics.90268.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Resp Crit Care Med. 2001;164(1971):1980. doi: 10.1164/ajrccm.164.10.2101140. [DOI] [PubMed] [Google Scholar]
- Bland RD, Mokres LM, Ertsey R, Jacobson BE, Jiang S, Rabinovitch M, Xu L, Shinwell ES, Zhang F, Beasley MA. Mechanical ventilation with 40% oxygen reduces pulmonary expression of genes that regulate lung development and impairs alveolar septation in newborn mice. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1099–L1110. doi: 10.1152/ajplung.00217.2007. [DOI] [PubMed] [Google Scholar]
- Bogue CW, Gross I, Vasavada H, Dynia DW, Wilson CM, Jacobs HC. Identification of Hox genes in newborn lung and effects of gestational age and retinoic acid on their expression. Am J Physiol. 1994;266:L448–L454. doi: 10.1152/ajplung.1994.266.4.L448. [DOI] [PubMed] [Google Scholar]
- Bustani P, Hodge R, Tellabati A, Li J, Pandya H, Kotecha S. Differential response of the epithelium and interstitium in developing human fetal lung explants to hyperoxia. Pediatr Res. 2006;59:383–388. doi: 10.1203/01.pdr.0000198774.79043.5c. [DOI] [PubMed] [Google Scholar]
- Coalson JJ. Pathology of the new bronchopulmonary dysplasia. Semin Neonatol. 2003;8:73–81. doi: 10.1016/S1084-2756(02)00193-8. [DOI] [PubMed] [Google Scholar]
- Dieperink HI, Blackwell TS, Prince LS. Hyperoxia and apoptosis in developing mouse lung mesenchyme. Pediatr Res. 2006;59:185–190. doi: 10.1203/01.pdr.0000196371.85945.3a. [DOI] [PubMed] [Google Scholar]
- Doyle LW, Faber B, Callanan C, Freezer N, Ford GW, Davis NM. Bronchopulmonary dysplasia in very low birth weight subjects and lung function in late adolescence. Pediatrics. 2006;118:108–114. doi: 10.1542/peds.2005-2522. [DOI] [PubMed] [Google Scholar]
- Galang CK, Hauser CA. Cooperative DNA binding of the human HoxB5 (Hox-2.1) protein is under redox regulation in vitro. Mol Cell Biol. 1993;13:4609–4617. doi: 10.1128/mcb.13.8.4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golpon HA, Gerace MW, Moore MD, Miller HL, Tuder RM, Voelkel NF. Hox genes in human lung:altered expression in primary pulmonary hypertension and emphysema. Am J Pathol. 2001;158:955–966. doi: 10.1016/S0002-9440(10)64042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graba Y, Aragnol D, Pradel J. Drosophila hox complex downstream targets and the function of homeotic genes. Bioessays. 1997;19:379–388. doi: 10.1002/bies.950190505. [DOI] [PubMed] [Google Scholar]
- Hamilton BE, Martin JA, Ventura SJ. Births: preliminary data for 2007. National Vital Stat Rep. 2009;57:1–21. [PubMed] [Google Scholar]
- Hershko AY, Kafri T, Fainsod A, Razin A. Methylation of Hoxa-5 and Hoxb-5 and its relevance to expression during mouse development. Gene. 2003;302:65–72. doi: 10.1016/S0378111902010910. [DOI] [PubMed] [Google Scholar]
- Higgins RD, Bancalari E, Willinger M, Raju TNK. Executive summary of the workshop on oxygen in neonatal therapies: controversies and opportunities for research. Pediatrics. 2007;119:790–796. doi: 10.1542/peds.2006-2200. [DOI] [PubMed] [Google Scholar]
- Hislop A. Developmental biology of the pulmonary circulation. Paediatr Respir Rev. 2005;6:35–43. doi: 10.1016/j.prrv.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Hombria JCG, Lovegrove B. Beyond homeoosis - Hox function in morphogenesis and organogenesis. Differentiation. 2003;71:461–476. doi: 10.1046/j.1432-0436.2003.7108004.x. [DOI] [PubMed] [Google Scholar]
- http://www.cbil.upenn.edu/cgi-bin/tess/tess (2010) TESS
- Inselman LS, Mellins RB. Growth and development of the lung. J Pediatr. 1981;98:1–15. doi: 10.1016/S0022-3476(81)80524-0. [DOI] [PubMed] [Google Scholar]
- Jobe AH. The new BPD. NeoReviews. 2006;7:e531–e545. doi: 10.1542/neo.7-10-e531. [DOI] [Google Scholar]
- Kinkead R, Leblanc M, Gulemetova R, Lalancette-Hebert M, Lemieux M, Mandeville I, Jeannotte L. Respiratory adaptations to lung morphological defects in adult mice lacking Hoxa-5 gene function. Pediatr Res. 2004;56:553–562. doi: 10.1203/01.PDR.0000139427.26083.3D. [DOI] [PubMed] [Google Scholar]
- Laptook AR, O’Shea TM, Shankaran S, Bhaskar B, Network NN. Adverse neurodevelopmental outcomes among extremely low birth weight infants with a normal head ultrasound: prevalence and antecedents. Pediatrics. 2005;115:673–680. doi: 10.1542/peds.2004-0667. [DOI] [PubMed] [Google Scholar]
- Lazarus P, Del Moral P, Ilves R, Mishra RK, Warburton D, et al. A perfusion-independent role of blood vessels in determining branching stereotypy of lung airways. Development. 2013;138:2359–2368. doi: 10.1242/dev.060723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelville I, Aubin J, Leblanc M, Lalancette-Hebert M, Janelle M-F, Tremblay GM, Jeannotte L. Impact of loss of Hoxa5 function on lung alveogenesis. Am J Pathol. 2006;169:1312–1327. doi: 10.2353/ajpath.2006.051333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniscalco WM, Watkins RH, O’Reilly J, Shea CP. Increased epithelial cell proliferation in very premature baboons with chronic lung injury. Am J Lung Cell Mol Physiol. 2002;283:L991–L1001. doi: 10.1152/ajplung.00050.2002. [DOI] [PubMed] [Google Scholar]
- Maniscalco WM, Watkins RH, Roper JM, Staversky R, O’reilly MA. Hyperoxic ventilated premature baboons have increased p53, oxidant DNA damage and decreased vegf expression. Pediatr Res. 2005;58:549–556. doi: 10.1203/01.pdr.0000176923.79584.f7. [DOI] [PubMed] [Google Scholar]
- McGrath-Morrow S, Rangasamy T, Cho C, Sussan T, Neptune E, Wise R, Tuder RM, Biswal S. Impaired lung homeostasis in neonatal mice exposed to cigarette smoke. Am J Respir Cell Mol Biol. 2008;38(4):393–400. doi: 10.1165/rcmb.2007-0104OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M, Clauss M, Lepple-Wienhues A, Waltenberger J, Augustin HG, Ziche M, Lanz C, Buttner M, Rziha HJ, Dehio C. A novel vascular endothelial growth factor encoded by Orf virus, VEGF-E, mediates angiogenesis via signalling through VEGFR-2 (KDR) but not VEGFR-1 (Flt-1) receptor tyrosine kinases. EMBO J. 1999;18(2):363–374. doi: 10.1093/emboj/18.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R, InDerRieden P, Hooiveld MHW, Durston AJ. Identifying Hox paralog groups by the PBX-binding region. TIG. 2000;16(2):66–67. doi: 10.1016/S0168-9525(99)01881-8. [DOI] [PubMed] [Google Scholar]
- Mujahid S, Nielsen HC, Volpe MV. miR-221 and miR130a regulate lung airway and vascular development. PLoS One. 2013;8:e55911. doi: 10.1371/journal.pone.0055911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’reilly MA. DNA damage and cell cycle checkpoints in hyperoxic lung injury: breaking to facilitate repair. Am J Physiol Lung Cell Mol Physiol. 2001;281:L291–L305. doi: 10.1152/ajplung.2001.281.2.L291. [DOI] [PubMed] [Google Scholar]
- Pan G, Simpson RU. Antisense knockout of Hoxb-4 blocks 1,25-dihydroxyvitamin D3 inhibition of c-myc expression. J Endocrin. 2001;169:153–159. doi: 10.1677/joe.0.1690153. [DOI] [PubMed] [Google Scholar]
- Rahman I, Yang SR, Biswas SK. Current concepts of redox signaling in the lungs. Antioxid Redox Signal. 2006;8(3–4):681–689. doi: 10.1089/ars.2006.8.681. [DOI] [PubMed] [Google Scholar]
- Rhoads K, Arderiu G, Charboneau A, Hansen SL, Hoffman W, Boudreau N. A role of Hoxa5 in regulating angiogenesis and vascular patterning. Lymphat Res Biol. 2005;3:240–252. doi: 10.1089/lrb.2005.3.240. [DOI] [PubMed] [Google Scholar]
- Shiraishi M, Sekiguchi A, Oates AJ, Terry MJ, Miyamoto Y. Hox gene clusters are hotspots of de novo methylation in CpG islands of human lung adenomas. Oncogene. 2002;21:3659–3662. doi: 10.1038/sj.onc.1205453. [DOI] [PubMed] [Google Scholar]
- Skalli O, Ropraz P, Trzeciak A, Benzonana G, Gillessen D, Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavkin HC, Johnson R, Oliver P, Bringas P, Don-Wheeler G, Mayo M, Whitsett JA. Lamellar body formation precedes pulmonary surfactant apoprotein expression during embryonic mouse lung development in vivo and in vitro. Differentiation. 1989;41:223–236. doi: 10.1111/j.1432-0436.1989.tb00751.x. [DOI] [PubMed] [Google Scholar]
- Stenmark KR, Abman SH. Lung vascular development: implications for the pathogenesis of bronchopulmonary dysplasia. Annu Rev Physiol. 2005;67:623–661. doi: 10.1146/annurev.physiol.67.040403.102229. [DOI] [PubMed] [Google Scholar]
- Ten Have-Opbroek AAW. Lung development in the mouse embryo. Exp Lung Res. 1991;17:111–130. doi: 10.3109/01902149109064406. [DOI] [PubMed] [Google Scholar]
- Thebaud B, Ladha F, Michelakis ED, Sawicka M, Thurston G, Eaton F, Hashimoto K, Harry G, Haromy A, Korbutt G, Archer SL. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury. Circulation. 2005;112:2477–2486. doi: 10.1161/CIRCULATIONAHA.105.541524. [DOI] [PubMed] [Google Scholar]
- Tyson JE, Wright LL, Oh W, Kennedy KA, Bele L, Ehrenkranz RA, Stoll BJ, Lemons JA, Stevenson DK, Bauer CR, Korones SB, Fanaroff AA, N. N. R. Network Vitamin A supplementation for extremely low birth weight infants. N Engl J Med. 1999;340:1962–1968. doi: 10.1056/NEJM199906243402505. [DOI] [PubMed] [Google Scholar]
- van Oostveen JW, Bijl JJ, Raaphorst FM, Walboomers JJM, Meijer CJLM. The role of homeobox genes in normal hematopoiesis and hematological malignancies. Leukemia. 1999;13:1675–1690. doi: 10.1038/sj.leu.2401562. [DOI] [PubMed] [Google Scholar]
- Volpe MV, Martin A, Vosatka RJ, Mazzoni CL, Nielsen HC. Hoxb-5 expression in the developing mouse lung suggests a role in branching morphogenesis and epithelial cell fate. Histochem Cell Biol. 1997;108:495–504. doi: 10.1007/s004180050190. [DOI] [PubMed] [Google Scholar]
- Volpe MV, Vosatka RJ, Nielsen HC. Hoxb-5 control of early airway formation during branching morphogenesis in the developing mouse lung. Biochim Biophys Acta. 2000;1475:337–345. doi: 10.1016/S0304-4165(00)00087-8. [DOI] [PubMed] [Google Scholar]
- Volpe MV, Pham L, Lessin M, Ralston SJ, Bhan I, Cutz E, Nielsen HC. Expression of Hoxb-5 during human lung development and in congenital lung malformations. Birth Defects Res Part A Clin Mol Teratol. 2003;67:550–556. doi: 10.1002/bdra.10086. [DOI] [PubMed] [Google Scholar]
- Volpe MV, Ramadurai SM, Pham LD, Nielsen HC. Hoxb-5 down regulation alters tenascin-C, FGF10 and Hoxb gene expression patterns in pseudoglandular period fetal mouse lung. Front Biosci. 2007;12:860–873. doi: 10.2741/2108. [DOI] [PubMed] [Google Scholar]
- Volpe MV, Wang KT, Nielsen HC, Chinoy MR. Unique spatial and cellular expression patterns of Hoxa5, Hoxb4 and Hoxb6 proteins in normal developing mouse lung are modified in pulmonary hypoplasia. Birth Defects Res Part A Clin Mol Teratol. 2008;82:571–584. doi: 10.1002/bdra.20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnik S, Klinkert M, Kurz H, Zoeller C, Heinke J, Wu Y, Bode C, Patterson C, Moser M. HoxB5 induces endothelial sprouting in vitro and modifies intussusceptive angiogenesis in vivo involving angiopoietin-2. Cardiovasc Res. 2009;83:558–565. doi: 10.1093/cvr/cvp133. [DOI] [PubMed] [Google Scholar]
- Wright CJ, Dennery PA. Manipulation of gene expression by oxygen: a primer from bedside to bench. Pediatr Res. 2009;66:3–10. doi: 10.1203/PDR.0b013e3181a2c184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Moser M, Bautch VL, Patterson C. Hoxb5 is an upstream transcriptional switch for differentiation of the vascular endothelium from precursor cells. Mol Cell Biol. 2003;23:5680–5691. doi: 10.1128/MCB.23.16.5680-5691.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Saegusa C, Schehr A, Grant S, Whitsett JA, Ikegami M. C/EBP{alpha} is required for pulmonary cytoprotection during hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2009;297(2):L286–L298. doi: 10.1152/ajplung.00094.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi TP, Dumont DJ, Conlon RA, Breitman ML, Rossant J. flk-1, an flt-related receptor tyrosine kinase is an early marker for endothelial cell precursors. Development. 1993;118(2):489–498. doi: 10.1242/dev.118.2.489. [DOI] [PubMed] [Google Scholar]