Abstract
BACKGROUND:
Allergic Broncho Pulmonary Aspergillosis (ABPA) is a rare disease characterized by an allergic inflammatory response to the colonization by aspergillus or other fungi in the airways. The aim was to study the clinical, radiological, and serological characteristics of patients of ABPA.
MATERIALS AND METHODS:
A prospective observational study of patients with breathlessness, chronic cough, blood eosinophilia, and infiltrates on chest X-ray were evaluated with serologic and allergic skin fungal tests using 15 common fungal antigens. Total of 24 patients were diagnosed as ABPA.
RESULTS:
Total 24 patients, 15 males (62%), 9 females (38%). Age range: 14-70 years, mean 49.13, standard deviation (SD) 14.12. Central bronchiectasis — sixteen patients, bronchocoele — one patient, consolidation — five patients, collapse with mucous plugging with areas of consolidation — three patients, one patient had bronchiectasis, consolidation with hemorrhagic pleural effusion. Fifty-eight percent of patients had received anti-tuberculosis medications prior to diagnosis. Serum total IgE varied from 340 to 18100 IU/mL. Two patients had IgE levels below 1,000 IU/mL. The mean decrease in Serum total IgE levels at the end of 1 month was 26.1% (range: 0.7-71.9%) and at the end of 2 months was 58.9% (range: 11.11-93.26%) (P value of 0.004). Two patients had skin sensitivity to fungal antigens other than aspergillus species.
CONCLUSION:
ABPA is a disease with varied clinical, radiological, and serological patterns. Serum IgE monitoring may be done at the end of 2 and 6 months. Further studies are required to simplify the diagnosis and treatment algorithms in resource-limited countries.
Keywords: Allergic, aspergillosis, bronchopulmonary, radiological, serological
Allergic Broncho Pulmonary Aspergillosis (ABPA) is a rare disease characterized by an allergic inflammatory response to the colonization by aspergillus or other fungi in the airways. It is estimated that ABPA complicates around 7-14% of cases of asthma.[1] ABPA should be ruled out in every case of uncontrolled asthma. Diagnostic criteria for ABPA include the presence of bronchial asthma, immediate skin test reactivity to A fumigatus, elevated serum IgE levels, total and A fumigatus-specific, pulmonary infiltrates (transient or fixed), central bronchiectasis, peripheral blood eosinophilia, and presence of serum precipitins against aspergillus antigen.[2] The currently employed diagnostic criteria have varying sensitivity and specificity.[3] A number of experts have proposed algorithmic approaches to the diagnosis of ABPA with some variations in the steps.[3]
Aim
The aim was to study the clinical, radiological and serological characteristics of patients of ABPA.
Materials and Methods
Design and setting: This was a prospective observational study of patients presenting to our specialty respiratory clinic either by referral from primary care physicians or directly presenting in the out patients department with complaints of breathlessness and chronic cough. This study was done from August 2008 till August 2012, presenting at a single outpatient center. A total of 2,295 patients were diagnosed with bronchial asthma and of these, 490 asthmatics were screened for suspicion of ABPA. Rests were dropped from the study for various reasons. This is a first study from western India on ABPA. In this study, we try to answer whether there are any regional variations in ABPA and whether frequent serologic monitoring as recommended in guidelines is mandated in resource limited settings.
Eligibility
All patients presenting in the outpatient department (OPD) with history and examination and fulfilling the diagnostic criteria suggesting ABPA were included in the study. Greenberger and Pattersons criteria were used to diagnose ABPA.[4] Those patients who did not wish to participate in the study were excluded Refer Table 1.
Table 1.
Diagnostic criteria of patients according to the Greenberger's criteria
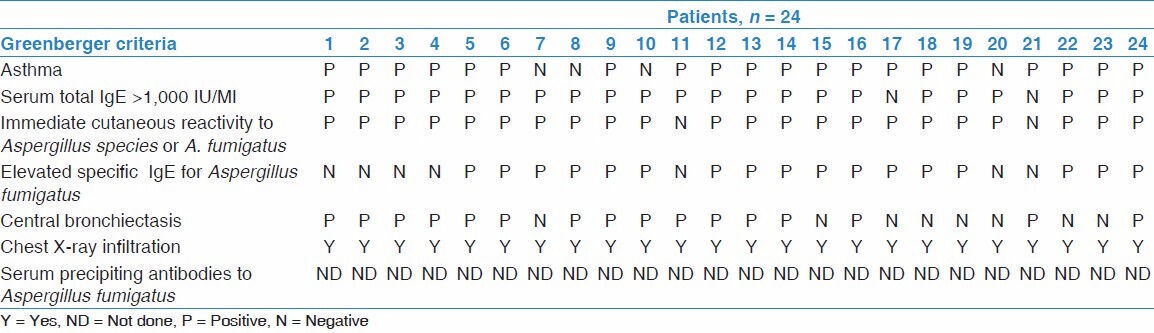
Data collection
Clinical data
Patient personal details — age, sex, education, occupation, family income, and addictions were recorded. Detailed history taking was done and complaints of cough, breathlessness, and sputum production were noted. General examination and respiratory system examinations were done. Clubbing was noted. Coarse crepts, palpable crepts were noted on respiratory system examination. Patients were specifically enquired about anti-tuberculosis medications in the past or present and the same was recorded.
Laboratory data
All patients were subjected for skin prick test using the standard 15 fungal allergens manufactured by Creative Drug Industries Limited, Mahape, Navi Mumbai. The various fungal allergens were Aspergillus fumigatus, Aspergillus niger, Aspergillus flavus, Fusarium solanii, Rhizopus nigricans, Aspergillus tamari, Curvularia lunata, Penicillium sp., Cladosporium herbarum, Alternaria alternate, Candida albicans, Tricoderma sp., Phoma tropicalis, Helminthosporium sp., Aspergillus versicolor. The readings were compared with controls using saline and histamine. The readings were measured according to Shivpuri's criteria.[5] The patients showing skin prick test positivity to any fungal antigen were subjected to Serum total IgE and High Resolution Computed tomography of the Chest (HRCT). Bronchiectasis was diagnosed on HRCT pattern.[6] Location of bronchiectasis was noted as central when it extended within medial one-half of the lung fields and peripheral when it extended outside the medial half. Patients were also subjected for pulmonary function testing (PFT) and specific IgE for Aspergillus fumigatus. We followed the algorithm recommended by Greenberger and Patterson.[3,7] Here, skin prick test was used as the screening test followed by serum total IgE. When these were positive, then specific IgE for Aspergillus fumigatus was done by Immunocap method and then HRCT chest was done to determine the abnormalities. Those patients with normal computed tomography (CT) chest were labeled as ABPA-serologic and excluded from the study (n = 96, 19.6%).
Treatment data
Patients diagnosed with ABPA were started on prednisolone or its equivalent, at a dose of 0.5 mg/kg per day for 1 month (maximum of 40 mg per day) followed by tapering of 5 mg every 2 weeks till 5 mg, given every other day after a month which was continued till 6 months.[8] After 6 months, patients were evaluated for symptomatic improvement and thereafter either stopped or continued for a further period of 3 months on alternate days. Itraconazole was given to patients whose serum IgE levels did not taper in 6 months and continued to have clinical symptoms (n = 2). These patients received 200 mg of itraconazole daily for a period of 3-6 months. All the patients were started on oral and inhaled bronchodilators, oral N acetyl cysteine, multivitamins, calcium, and protein supplements. Patients were vaccinated with influenza and pneumococcal vaccinations. Patients were advised follow-up visits at monthly intervals to record for symptoms and were noted for compliance of therapy, any exacerbations, adverse events due to medications, and any non-institutional consultations for exacerbations or any adverse events. Patients were followed for about 2 years and recorded for outcome: Remission, relapse, corticosteroid dependence, and end-stage fibrotic. All visits were separately recorded. Serum IgE was recorded at monthly intervals for 2 months and thereafter bimonthly till the end of 6 months. In our study, one patient had pleural effusion [Figures 1a and b].
Figure 1.

(a) HRCT of patient with ABPA showing collapse consolidation in right middle lobe with both lower lobes showing dilated mucous filled bronchi. (b) HRCT of same patient after a gap of 5 years showing right middle lobe bronchiectasis with complete collapse of left lung with moderate pleural effusion
Statistical analysis
Statistical analysis was done with Windows Statistical Package for the Social Sciences (SPSS) version 18. Descriptive statistics were completed using medians and means, as appropriate. P values (determined by χ2 or Fisher's exact tests) were reported; alpha was inferred at 0.05. Analysis of variance (ANOVA) was used to compute the values of serum IgE over various months and the decline of the IgE over the same period. An informed consent was obtained from all patients.
Results
Total numbers of patients were 24 (4.8% of the total asthmatics screened). There were 15 males (62%) and 9 females (38%). Age range: 14-70 years, mean 49.13, standard deviation (SD) 14.12. The mean duration of asthma in our study was 14.79 years. Duration of cough and breathlessness varied from 1-39 years. Clubbing was noted in 25% patients (n = 6). In our study, four patients had no prior history of asthma. Of these, three of these patients had reduction in Forced Expiratory Flow (FEF)25-75% on spirometry with partial bronchodilator reversibility. Twenty two patients had skin sensitivity to Aspergillus fumigatus alone. Two patients had skin sensitivity to antigens other than Aspergillus fumigatus. They were sensitive for helminthosporium, alternaria, and fusarium solanii. One patient tested positive for both Aspergillus fumigatus and Aspergillus flavus. A total of 58.3% patients received anti-tuberculosis treatment in our study (n = 14) before the diagnosis of ABPA. HRCT features were as follows: Central bronchiectasis alone was seen in 67% of patients (n = 16). Consolidation was seen in 20.8% of patients (n = 5) in addition to central bronchiectasis. Bronchocoele was seen in one patient. A total of 12.5% patients had collapse of a segment or lobe with mucous plugging with patchy areas of consolidation (n = 3) [Figure 2]. There was no evidence of bronchiectasis in these patients. One-third of our patients showed other radiologic features (ABPA-CB-ORF) with or without central bronchiectasis (n = 8). One patient had unilateral hemorrhagic pleural effusion in addition to bronchiectasis and consolidation [Figures 1a and b] which grew Aspergillus fumigatus on fungal culture.
Figure 2.

HRCT of patient with ABPA showing complete left lung collapse due to mucous plug obstruction
Serum total IgE varied from 340 IU/mL to 18,100 IU/mL on presentation. Two patients had IgE levels below 1,000 IU/mL on presentation. In our study, patients with ABPA-CB- ORF (other radiologic features) were more likely to have higher total IgE values as compared to ABPA-CB (central bronchiectasis alone). The mean total IgE in ABPA-CB-ORF was 11,740 IU/mL while in ABPA-CB was 5,588 IU/mL. This was statistically analyzed and significant difference was found in the values (P value of 0.02). The average forced expiratory volume in 1 second (FEV1) was 61%, range 33-82%. Specific IgE for Aspergillus fumigatus was done in all patients and was positive in 17 out of 24 patients (70.8%). Minimum level of specific IgE was Class IV, i.e., 3.90 kU/l or greater. Seven patients had a negative specific IgE. The serum IgE values over 6 months were computed and the differences observed. Refer Table 2. The mean IgE at the end of 1 month was 4,425 IU/mL (SD 4,218), at the end of 2 months was 1,821 IU/mL (SD 1,215), and that at the end of 6 months was 1,911 IU/mL (SD 1,106). The mean decrease in serum total IgE levels at the end of 1 month was 26.1% (range: 0.7-71.9%, SD 24.93), at the end of 2 months was 58.9% (range: 11.11-93.26%, SD 24.39), and at the end of 6 months was 60.8% (range: −20-95%, SD 39.64; P value of 0.03). Refer Table 3. In our study, 21% of patients had a drop of more than 35% (n = 5) from the baseline IgE levels at the end of 1 month and 83% of patients at the end of 2 months (n = 21). At the end of 6 months, only two patients did not have significant (>35%) decline in serum IgE levels. Itraconazole was started in these two patients. These two patients improved symptomatically and serum IgE levels decreased by more than 35% from baseline at the end of 1 year.
Table 2.
Analysis of Serum IgE values over 6-month period
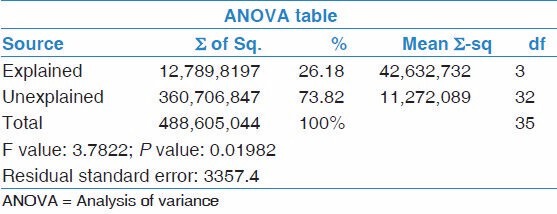
Table 3.
Decline in serum IgE values over 6 months

Complications were as follows: One patient developed pulmonary tuberculosis. One death was seen because of gram-negative bacterial (pseudomonal) sepsis.
Discussion
We reviewed all the original articles by Indian authors[6,9,10,11,12,13,14,15,16,17,18] as well as some of the international[19,20,21,22,23] original articles on ABPA and compared with our study. ABPA is seen commonly between third and fifth decades of life[3,19] and rarely in infants.[20] ABPA is predominantly seen in asthmatics with duration of asthma varying from 8.8 years to 12.5 years.[6,9,10,11,12,13,14,15,16,17,18] Rarely, ABPA is also seen in non-asthmatics. In two different studies by Behera et al.[10] and Chakraborathi et al.[14] , a total of 12 patients have been reported with no prior history of asthma. Glancy et al. in 1981 have reported 11 cases of ABPA without asthma (n = 42).[23] Hogan[24] , Anirban Sarkar et al.,[9] and Glancy et al.[23] have reported sensitization to fungi other than Aspergillus fumigatus. Hence, the name ABPA is a misnomer. ABPM is a more appropriate term for this disease. Misdiagnosis of tuberculosis varied from 29% in the study by Chakraborathi et al.[14] to 38.4% in Kumar et al.[18] to as high as 91% in the study by Prasad et al.[16] This shows that the awareness of this disease among primary care physicians is still very low all over the country. Central bronchiectasis is the commonest presentation as in various studies (69-100% of the cases)[6,9,10,11,12,13,14,15,16,17,18,21,25,26] . Rarely, pleural effusions have been reported in literature. Mintzer et al.[22] reported a single patient with pleural effusion out of 20 total patients. Agarwal et al.[6] also noted pleural effusions to be a relatively rare presentation in ABPA.
In the landmark study by Ricketti and Greenberger et al.,[27] the serum IgE ranged between 1,527−5,8000 ng/mL. The mean IgE in varied from 1,020 to 14,300 IU/mL in the study by Kumar et al.[18] and 6,265 IU/mL in the Aggarwal et al.[6] study. Ricketti et al. have suggested a cut off greater than 417 IU/mL for diagnosis of ABPA.[27] Mahdavinia and Grammer[28] have reported the controversy on the threshold for total IgE with some centers using 1,000 IU/mL and others using 417 IU/mL. In the latest study by Kumar et al.,[18] higher mean IgE value (14,300 IU/mL) was seen in the ABPA-CB-ORF group as compared to ABPA CB (1,020 IU/mL). Serial IgE monitoring has been suggested by various authors to monitor control of the disease.[1,8] Chupp has recommended monthly monitoring of IgE.[1] Ricketti and Greenberger have recommended monitoring every 8 weeks[2,27] . However, there are no clear consensuses on the frequency of serum IgE level monitoring. In resource-limited settings intermittent checking of serum total IgE levels may be cost-effective. Agarwal et al. have suggested that checking serum IgE levels at the end of 6 weeks did not predict clinical outcome.[29] To our best knowledge, there are no studies which have compared the declines in serum IgE values over 6 months. We propose that serum IgE may be done at the end of 2 months to detect immunological control of the disease and at the end of 6 months to detect treatment failures and steroid dependence.
Itraconazole is a well-documented drug used in ABPA as a steroid sparing agent. Stevens et al. have shown the efficacy of itraconazole in steroid-dependent patients in a randomized controlled trial.[30] However, in treatment naïve patients the guidelines for the use of azoles is still not clear. Mahdavinia and Grammer[28] and other authors[2,31] have recommended the use of itraconazole only after a trial of steroids has proven to be insufficient. Itraconazole use is still prohibitive due to the high cost and potential adverse effects of this drug. Chishimba et al.[32] have shown in their recent retrospective study that itraconazole is associated with 40% treatment failure and newer azoles may be effective in controlling asthma and reduction in IgE levels.
Conclusion
ABPA is a disease with varied clinical, radiological, and serological patterns. Patients with other radiologic features have a severe clinical and serological disease. Allergic bronchopulmonary mycosis is an appropriate term for the disease. Serum IgE monitoring may be done at the end of 2 months and 6 months. Further studies are required to simplify the diagnosis and treatment algorithms in resource limited countries.
Limitations
This study was a descriptive study in a small cohort of patients. A large number of patients data needs to be analyzed before a guideline be made on serial monitoring of serum IgE. Newer treatments for ABPA need to be undertaken as case-control studies with a large number of patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Chupp G, Rochester CL. Allergic Bronchopulmonary Aspergillosis (Mycosis) In: Fishman PA, editor. Fishman's Pulmonary Diseases and Disorders. 4th ed. Vol. 1. New York: Mac-Graw Hill; 2008. pp. 837–44. [Google Scholar]
- 2.Greenberger PA. Allergic bronchopulmonary aspergillosis. In: Grammer LC, Greenberger PA, editors. Patterson's Allergic Diseases. Philadelphia: Lippincott Williams and Wilkins; 2002. pp. 529–54. [Google Scholar]
- 3.Varkey B. Allergic bronchopulmonary aspergillosis- Clinical perspectives. Immunol Allergy Clin North Am. 1998;18:480–503. [Google Scholar]
- 4.Greenberger PA, Patterson R. Diagnosis and management of allergic bronchopulmonary aspergillosis. Ann Allergy. 1986;56:444–8. [PubMed] [Google Scholar]
- 5.Shivpuri DN. Comparative evaluation of sensitivity of common methods of diagnostic antigen tests in patients of respiratory allergy. Indian J Chest Dis. 1962;4:102–4. [Google Scholar]
- 6.Agarwal R, Gupta D, Aggarwal AN, Behera D, Jindal SK. Allergic bronchopulmonary aspergillosis: Lessons from 126 patients attending a chest clinic in north India. Chest. 2006;130:442–8. doi: 10.1378/chest.130.2.442. [DOI] [PubMed] [Google Scholar]
- 7.Patterson R, Greenberger PA. Potential errors in the diagnosis and management of allergic bronchopulmonary aspergillosis. In: Patterson R, Greenberger PA, editors. Allergic Bronchopulmonary Aspergillosis. Providence: Oceanside Publications; 1995. p. 29. [Google Scholar]
- 8.Fink JN. Therapy of allergic bronchopulmonary aspergillosis. Immunol Allergy Clin North Am. 1998;18:655–60. [Google Scholar]
- 9.Sarkar A, Mukharjee A, Ghoshal AG, Kundu S, Mitra S. Occurrence of allergic bronchopulmonary mycosis in patients wth asthma: An eastern India experience. Lung India. 2010;27:212–6. doi: 10.4103/0970-2113.71949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behera D, Guleria R, Jindal SK, Chakrabarti A, Panigrahi D. Allergic bronchopulmonary aspergillosis: A retrospective study of 35 cases. Indian J Chest Dis Allied Sci. 1994;36:173–9. [PubMed] [Google Scholar]
- 11.Bedi RS. Allergic bronchopulmonary aspergillosis: Review of 20 cases. Indian J Chest Dis Allied Sci. 1994;36:181–6. [PubMed] [Google Scholar]
- 12.Kumar R. Allergic bronchopulmonary aspergillosis: Review of 29 cases. Indian J Tuberc. 2000;47:237–9. [Google Scholar]
- 13.Kumar R, Gaur SN. Prevalence of allergic bronchopulmonary aspergillosis in patients with bronchial asthma. Asian Pac J Allergy Immunol. 2000;18:181–5. [PubMed] [Google Scholar]
- 14.Chakrabarti A, Sethi S, Raman DS, Behera D. Eight-year study of allergic bronchopulmonary aspergillosis in an Indian teaching hospital. Mycoses. 2002;45:295–9. doi: 10.1046/j.1439-0507.2002.00738.x. [DOI] [PubMed] [Google Scholar]
- 15.Shah A, Panchal N, Panjabi C. Allergic bronchopulmonary aspergillosis: A review from India. Allergy Clin Immunol Int. 2003;1:S104. [Google Scholar]
- 16.Prasad R, Garg R, Sanjay A, Shukla D. Allergic bronchopulmonary aspergillosis: A review of 42 patients from a tertiary care center in India. Lung India. 2009;26:38–40. doi: 10.4103/0970-2113.48895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghosh T, Dey A, Biswas D, Chatterjee S, Haldar N, Maiti PK. Aspergillus hypersensitivity and allergic bronchopulmonary aspergillosis among asthma patients in eastern India. J Indian Med Assoc. 2010;108:863–5. [PubMed] [Google Scholar]
- 18.Kumar R, Goel N. Allergic bronchopulmonary aspergillosis: A clinico-serological correlation with radiologic profile. J Asthma. 2013;50:759–63. doi: 10.3109/02770903.2013.796973. [DOI] [PubMed] [Google Scholar]
- 19.Malo JL, Hawkins R, Pepys J. Studies in chronic allergic bronchopulmonary aspergillosis: Clinical and physiological findings. Thorax. 1977;32:254–61. doi: 10.1136/thx.32.3.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imbeau SA, Cohen M, Reed CE. Allergic bronchopulmonary aspergillosis in infants. Am J Dis Child. 1977;131:1127–30. doi: 10.1001/archpedi.1977.02120230073013. [DOI] [PubMed] [Google Scholar]
- 21.Angus RM, Davies ML, Cowan MD, Mcsharry C, Thomson NC. Computed tomographic scanning of the lung in patients with allergic bronchopulmonary aspergillosis and in asthmatic patients with a positive skin test to Aspergillus fumigatus. Thorax. 1994;49:586–9. doi: 10.1136/thx.49.6.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mintzer RA, Rogers LF, Kruglik GD, Rosenberg M, Neiman HL, Patterson R. The spectrum of radiologic findings in allergic bronchopulmonary aspergillosis. Radiology. 1978;127:301–7. doi: 10.1148/127.2.301. [DOI] [PubMed] [Google Scholar]
- 23.Glancy JJ, Elder JL, McAleer R. Allergic bronchopulmonary fungal disease without clinical asthma. Thorax. 1981;36:345–9. doi: 10.1136/thx.36.5.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hogan MB. Other allergic bronchopulmonary mycoses. In: Patterson R, Greenberger PA, Roberts M, editors. Allergic Bronchopulmonary Aspergillosis. Providence: OceanSide Publications; 1995. pp. 57–60. [Google Scholar]
- 25.Agarwal R, Khan A, Garg M, Aggarwal AN, Gupta D. Chest radiographic and computed tomographic manifestations in allergic bronchopulmonary aspergillosis. World J Radiol. 2012;4:141–50. doi: 10.4329/wjr.v4.i4.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta RK, Chandr A, Gautam PB. Allergic bronchopulmonary aspergillosis - A clinical review. J Assoc Physicians India. 2012;60:46–51. [PubMed] [Google Scholar]
- 27.Ricketti AJ, Greenberger PA, Patterson R. Serum IgE as an important aid in management of allergic bronchopulmonary xaspergillosis. J Allergy Clin Immunol. 1984;74:68–71. doi: 10.1016/0091-6749(84)90089-7. [DOI] [PubMed] [Google Scholar]
- 28.Mahdavinia M, Grammer LC. Management of allergic bronchopulmonary aspergillosis: A review and update. Ther Adv Respir Dis. 2012;6:173–87. doi: 10.1177/1753465812443094. [DOI] [PubMed] [Google Scholar]
- 29.Agarwal R, Gupta D, Aggarwal AN, Saxena AK, Saikia B, Chakrabarti A, et al. Clinical significance of decline in serum IgE levels in allergic bronchopulmonary aspergillosis. Respir Med. 2010;104:204–10. doi: 10.1016/j.rmed.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Stevens DA, Schwartz HJ, Lee JY, Moskovitz BL, Jerome DC, Catanzaro A, et al. A randomized trial of itraconazole in allergic bronchopulmonary aspergillosis. N Engl J Med. 2000;342:756–62. doi: 10.1056/NEJM200003163421102. [DOI] [PubMed] [Google Scholar]
- 31.Wark PA, Gibson PG, Wilson AJ. Azoles for allergic bronchopulmonary aspergillosis associated with asthma. Cochrane Database Syst Rev. 2004:CD001108. doi: 10.1002/14651858.CD001108.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chishimba L, Niven RM, Cooley J, Denning DW. Voriconazole and posaconazole improve asthma severity in allergic bronchopulmonary aspergillosis and severe asthma with fungal sensitization. J Asthma. 2012;49:423–33. doi: 10.3109/02770903.2012.662568. [DOI] [PubMed] [Google Scholar]


