Abstract
INTRODUCTION:
The differential diagnosis of sarcoidosis creates a challange due to tuberculosis also having lung and lymph node involvement. Because both diseases show granulomatous inflammation, it may not be possible to distinguish tuberculosis and sarcoidosis in pathological specimens. As a result of the complexity in the differential diagnosis of sarcoidosis and tuberculosis, new markers for differentiation are being investigated.
OBJECTIVE:
The aim of our study is to investigate the value of neutrophil/lymphocyte ratio (NLR) as a possible marker in differentiating sarcoidosis and tuberculosis.
MATERIALS AND METHODS:
In our study, 51 acid-fast bacilli (AFB) positive and/or culture-positive patients with pulmonary tuberculosis, 40 patients with biopsy-proven sarcoidosis and a control group consisting of 43 patients were included. In our study, information was collected retrospectively based on hospital records.
RESULTS:
Leukocyte and neutrophil counts, NLR, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) were significantly higher, and albumin was significantly lower in the tuberculosis group compared with sarcoidosis (for all parameters P < 0.001). The most appropriate cut-off value of NLR to distinguish tuberculosis from sarcoidosis was determined as 2.55. For this cut-off value of NLR there was 79% sensitivity, 69% specificity, 73% positive predictive value (PPV), 75% negative predictive value (NPV), and area under the curve (AUC) was 0.788. For differentiation of sarcoidosis from tuberculosis, accuracy of the NLR test according to this cut-off value was found as 76%.
CONCLUSION:
NLR as a little known marker in respiratory medicine was found to be supportive in differentiation of tuberculosis and sarcoidosis. More studies on this issue is needed.
Keywords: Granulomatous inflammation, neutrophil-lymphocyte ratio, sarcoidosis, tuberculosis
Tuberculosis is a common infectious disease in the world. Therefore, it has a very important place in differential diagnosis of pulmonary and extrapulmonary granulomatous diseases. In order to diagnose the disease, pathogen are required to demonstrate. Because this is not always possible, the diagnosis can be made based on clinical, pathological and radiological findings. The differential diagnosis of sarcoidosis creates a challange due to tuberculosis also having lung and lymph node involvement. Because both diseases show granulomatous inflammation, it may not be possible to distinguish tuberculosis and sarcoidosis in pathological specimens. In sarcoidosis, symptoms such as fever, fatigue, weight loss and cough may be present, similar to tuberculosis, thus making clinical differentiation of sarcoidosis and tuberculosis difficult.[1] As a result of the complexity in the differential diagnosis of sarcoidosis and tuberculosis, new markers for differentiation are being investigated.
The neutrophil/lymphocyte ratio (NLR) is determined by dividing the absolute count of neutrophils by the number of lymphocytes in the complete blood count. NLR has emerged as a new marker of inflammation.[2,3,4,5] Studies are available showing the relationship between NLR and prognosis in lung cancer and colorectal cancer.[6,7,8,9] In addition, high-NLR in acute coronary syndrome and patients undergoing coronary invasive procedures has been shown to be associated with poor prognosis.[10,11] Studies in the field of chest diseases are usually focused on lung cancer. There are few studies about chronic obstructive pulmonary disease (COPD), tuberculosis and pneumonia.[12,13,14] The aim of our study is to investigate the value of NLR as a possible marker in differentiating sarcoidosis and tuberculosis.
Materials and Methods
In our study 51 acid-fast bacilli (AFB) positive and/or culture-positive patients with pulmonary tuberculosis, 40 patients with biopsy-proven sarcoidosis and a control group consisting of 43 patients were included. All patients diagnosed as pulmonary tuberculosis and sarcoidosis in our Pulmonology clinic in 2012-2013 were included. Exclusion criteria were defined as: Acute coronary syndrome, presence of active malignancy, hematologic disease, steroid use, having immunosuppressive therapy, the presence of other active infections. Forty-three asymptomatic patients who were admitted to the internal medicine out-patient clinic for routine health control were included as the control group. Patients who had infectious, inflammatory and malignant pathologies were excluded.
In our study, information was collected retrospectively based on hospital records. We recorded the patients' age, sex, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), complete blood count, and serum albumin levels. The NLR was obtained by dividing the absolute number of neutrophils by lymphocytes in complete blood count. An automatic blood count device was used for the complete blood count. Pre-treatment acute-phase reactant levels were recorded from medical records.
Statistical analyses were performed using the SPSS (Statistical Package for Social Sciences) 15.0 software package. Descriptive values were given as mean and standard deviation. Categorical variables were expressed as the number of cases and the percentage value. Continuous variables were analyzed using Kolmogorov-Smirnov and Shapiro-Wilk tests to determine whether there was normal distribution. The Student's t-test and Mann-Whitney-U test were used depending on the situation of the variables i. e. normally distributed or not. The comparison of categorical variables were performed using Chi-square and Fisher's exact tests. Spearman's correlation test was used to analyse the relationship between NLR and other inflammatory markers. In order to determine the cut-off value of NLR, ROC (receiver operating characteristics) curve analysis was used. Statistical significance was set as P < 0.05.
Results
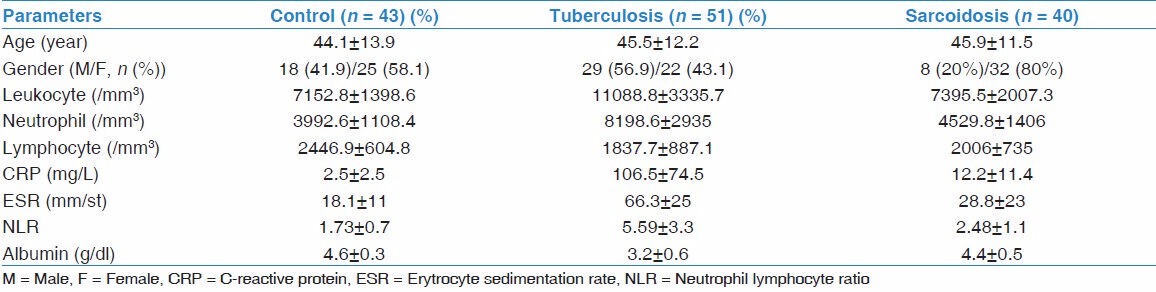
A total of 134 patients were included into the study. Tuberculosis group consisted of 51 patients. Twenty-nine (56.9%) of them were men and 22 (43.1%) were female. The mean age was 45.5 ± 12.2 years. The sarcoidosis group consisted of 40 patients. Of these, 8 patients (20%) were men and 32 (80%) were female. The mean age of the sarcoidosis group was 45.9 ± 11.5 years. The control group consisted of 43 patients, 18 patients (41.9%) were men and 25 (58.1%) were female. The mean age was 44.1 ± 13.9 years. The mean age was similar in all three groups (P > 0.05). The tuberculosis and control groups were similar in terms of sex (P > 0.05); however, the majority of the sarcoidosis group were female. The demographic and laboratory characteristics of the patients are summarized in Table 1.
Table 1.
Demographic and laboratory characteristics of groups
In the evaluation of patients with tuberculosis, NLR showed positive correlation with CRP (r = 0.500, P < 0.001). Also, there was a positive correlation between CRP and ESR in this group (r = 0.500, P = 0.009). There were no further correlations between other inflammatory markers in the tuberculosis group. In the evaluation of patients with sarcoidosis, a positive correlation was found between CRP and ESR (r = 0.506, P = 0.008). There were no other correlations between inflammatory markers in the sarcoidosis group.
When the tuberculosis and control groups were compared with each other, a statistically significant difference was found between the groups in terms of leukocyte, neutrophil, lymphocyte counts, ESR, CRP, serum albumin and NLR (for all parameters P < 0.001). Neutrophils, white blood cells (WBC), ESR, and CRP were higher in the tuberculosis group. On the other hand, lymphocytes and albumin were lower in this group compared with controls. The NLR was significantly higher in the tuberculosis group in parallel with the increase in neutrophils and the decline in lymphocytes (P < 0.001) [Table 1]. In order to distinguish cases of tuberculosis from the control group, the most appropriate cut-off value was 2.16. For this cut-off value, NLR had sensitivity of 88% and specificity of 80%, positive predictive value (PPV) of 82%, negative predictive value (NPV) of 87% and area under the curve (AUC) was 0.921. According to this cut-off value, accuracy of the NLR test for distinguishing tuberculosis from the control group was 84%. See Table 2 for other markers.
Table 2.
ROC analysis results and accuracy rates of NLR and other inflammatory markers in detection of tuberculosis among tuberculosis and control groups
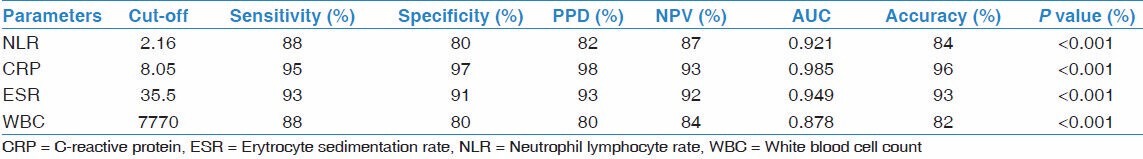
When the sarcoidosis and control groups were compared, neutrophil count, NLR and CRP were significantly higher, and lymphocytes were significantly lower in the sarcoidosis group (respectively P = 0.04, P < 0.001, P < 0.001, P = 0.005). There were no statistically significant differences between the groups for total leukocyte count, ESR and albumin (P > 0.05) [Table 1]. The value of 1.88 was determined as the most appropriate cut-off value for NLR for the distinction of sarcoidosis and controls. For this cut-off value, NLR had a sensitivity of 73%, specificity of 74%, PPV of 68%, NPV of 71%, and AUC was 0.790. The accuracy of NLR test for this cut-off value was 70% for distinction of sarcoidosis and controls. See Table 3 for the other markers.
Table 3.
ROC analysis results and accuracy rates of NLR and other inflammatory markers in detection of sarcoidosis among sarcoidosis and control groups
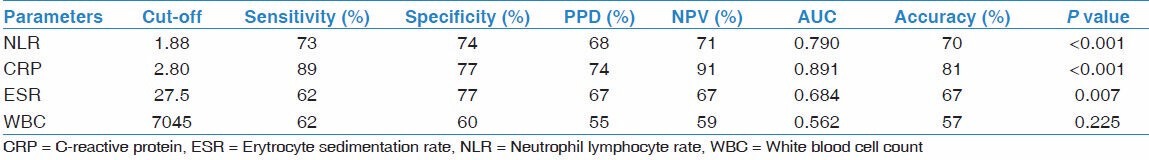
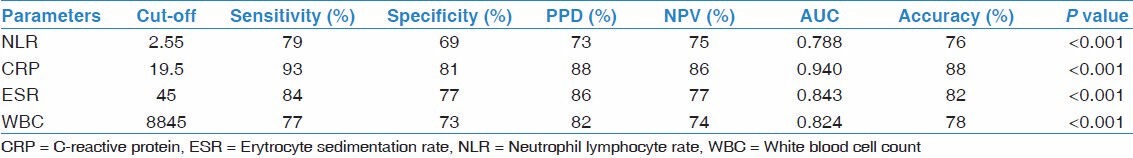
Leukocyte and neutrophil counts, NLR, ESR, and CRP were significantly higher, and the albumin was significantly lower in the tuberculosis group compared with sarcoidosis (for all parameters P < 0.001). No significant difference was observed in the lymphocyte count in this comparison (P > 0.05) [Table 1]. The most appropriate cut-off value of NLR to distinguish tuberculosis from sarcoidosis was determined as 2.55. For this cut-off value of NLR there was 79% sensitivity, 69% specificity, 73% PPV, 75% NPV, and AUC was 0.788. For differentiation of sarcoidosis from tuberculosis, accuracy of the NLR test according to this cut-off value was found as 76%. See Table 4 for the other markers.
Table 4.
ROC analysis results and accuracy rates of NLR and other inflammatory markers in detection of tuberculosis among sarcoidosis and tuberculosis groups
Discussion
Many publications have been written on the relationship between prognosis and NLR as a marker of inflammation. The value of NLR has been studied in cardiovascular disease, chronic renal disease, malignancies, osteoporosis and Alzheimer's disease.[6,7,8,9,10,11,15,16,17] However, the most popular area of study in pulmonary medicine is its relationship with prognosis of lung cancer and mesothelioma.[6,7,18,19] In non-small cell lung cancer and mesothelioma, high NLR was associated with poor prognosis. In other areas of respiratory medicine, there are very few studies. One of these areas is COPD. In the study of Gunay et al., NLR has been shown to be higher in acute exacerbation of COPD compared with both stable COPD and healthy controls. In this study, NLR was correlated with CRP in COPD and it was suggested that NLR could be used as a new marker of inflammation in COPD.[12] Few studies are available evaluating the value of NLR in infectious lung diseases. According to the study of Yoon et al., NLR can be used for the discrimination of tuberculosis and community acquired pneumonia.[13] De Jager et al.,'s study suggested that NLR calculated on admission to the emergency room provided a better prediction for the course of community acquired pneumonia.[14]
Tuberculosis/sarcoidosis distinction is very difficult in some cases and there is a need for new markers in this area. Sometimes it is not even possible to distinguish clinically or pathologically. In this situation, a patient should be started on anti-tuberculosis therapy and closely monitored. Our study was conducted to determine if NLR contributes to the differentiation of tuberculosis and sarcoidosis, and if NLR value differs in disease groups with respect to the control group.
Gunay et al., showed a positive correlation between CRP and NLR in COPD patients.[12] Similarly, there was a positive correlation between NLR and CRP in the tuberculosis group of our study. The NLR in tuberculosis and sarcoidosis patients were significantly higher than the control group. In the comparison of tuberculosis and sarcoidosis, a cut-off value of NLR ≥ 2.55 can differentiate tuberculosis with an accuracy rate of 76%.
The neutrophil/lymphocyte ratio is a marker that can be calculated easily from a routine complete blood count. It does not introduce additional workload or cost to the clinician or the laboratory. It is easily repeatable. With present and ongoing studies, NLR use is becoming increasingly common in malignant and non-malignant diseases. Its success in predicting prognosis is emphasized in many studies.[6,7,8,9,10,11] Besides, its beneficial role in diagnosis needs more studies. Limitations of our study are being retrospective, based on hospital archive, not having a large sample size and giving a 69% specificity for distinguishing tuberculosis and sarcoidosis. In order to evaluate clinical relevance of NLR in sarcoidosis and tuberculosis, more studies with larger sample size are needed. Our study leads a new discussion area on role of NLR in granulomatous lung diseases.
In conclusion, NLR has attracted attention as a new inflammatory marker. Different usage areas are being explored. To date, the difficulty in differentiating sarcoidosis and tuberculosis remains. In our study, NLR as a little known marker in respiratory medicine was found to be supportive in differentiation of tuberculosis and sarcoidosis. More studies on this issue are needed.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Telenti A, Hermans PE. Idiopathic granulomatosis manifesting as fever of unkown origin. Mayo Clin Proc. 1989;64:44–50. doi: 10.1016/s0025-6196(12)65302-6. [DOI] [PubMed] [Google Scholar]
- 2.Jilma B, Blann A, Pernerstorfer T, Stohlawetz P, Eichler HG, Vondrovec B, et al. Regulation of adhesion molecules during human endotoxemia. No acute effects of aspirin. Am J Respir Crit Care Med. 1999;159:857–63. doi: 10.1164/ajrccm.159.3.9805087. [DOI] [PubMed] [Google Scholar]
- 3.Dionigi R, Dominioni L, Benevento A, Giudice G, Cuffari S, Bordone N, et al. Effects of surgical trauma of laparoscopic vs. open cholecystectomy. Hepatogastroenterology. 1994;41:471–6. [PubMed] [Google Scholar]
- 4.O'Mahony JB, Palder SB, Wood JJ, McIrvine A, Rodrick ML, Demling RH, et al. Depression of cellular immunity after multiple trauma in the absence of sepsis. J Trauma. 1984;24:869–75. doi: 10.1097/00005373-198410000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Zahorec R. Ratio of neutrophil to lymphocyte counts - rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102:5–14. [PubMed] [Google Scholar]
- 6.Sarraf KM, Belcher E, Raevsky E, Nicholson AG, Goldstraw P, Lim E. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2009;137:425–8. doi: 10.1016/j.jtcvs.2008.05.046. [DOI] [PubMed] [Google Scholar]
- 7.Cedrés S, Torrejon D, Martínez A, Martinez P, Navarro A, Zamora E, et al. Neutrophil to lymphocyte ratio (NLR) as an indicator of poor prognosis in stage IV non-small cell lung cancer. Clin Transl Oncol. 2012;14:864–9. doi: 10.1007/s12094-012-0872-5. [DOI] [PubMed] [Google Scholar]
- 8.Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181–4. doi: 10.1002/jso.20329. [DOI] [PubMed] [Google Scholar]
- 9.Halazun KJ, Aldoori A, Malik HZ, Al-Mukhtar A, Prasad KR, Toogood GJ, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. Eur J Surg Oncol. 2008;34:55–60. doi: 10.1016/j.ejso.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 10.Tamhane UU, Aneja S, Montgomery D, Rogers EK, Eagle KA, Gurm HS. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. 2008;102:653–7. doi: 10.1016/j.amjcard.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Duffy BK, Gurm HS, Rajagopal V, Gupta R, Ellis SG, Bhatt DL. Usefulness of an elevated neutrophil to lymphocyte ratio in predicting long-term mortality after percutaneous coronary intervention. Am J Cardiol. 2006;97:993–6. doi: 10.1016/j.amjcard.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 12.Gunay E, Sarýnç Ulasli S, Akar O, Ahsen A, Gunay S, Koyuncu T, et al. Neutrophil-to-lymphocyte ratio in chronic obstructive pulmonary disease: A retrospective study. Inflammation. 2014;37:374–80. doi: 10.1007/s10753-013-9749-1. [DOI] [PubMed] [Google Scholar]
- 13.Yoon NB, Son C, Um SJ. Role of the neutrophil-lymphocyte count ratio in the differential diagnosis between pulmonary tuberculosis and bacterial community-acquired pneumonia. Ann Lab Med. 2013;33:105–10. doi: 10.3343/alm.2013.33.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Jager CP, Wever PC, Gemen EF, Kusters R, van Gageldonk-Lafeber AB, van der Poll T, et al. The neutrophil-lymphocyte count ratio in patients with community-acquired pneumonia. PLoS One. 2012;7:e46561. doi: 10.1371/journal.pone.0046561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahsen A, Ulu MS, Yuksel S, Demir K, Uysal M, Erdogan M, et al. As a new inflammatory marker for familial Mediterranean fever: Neutrophil-to-lymphocyte ratio. Inflammation. 2013;36:1357–62. doi: 10.1007/s10753-013-9675-2. [DOI] [PubMed] [Google Scholar]
- 16.Núñez J, Núñez E, Bodí V, Sanchis J, Miñana G, Mainar L, et al. Usefulness of the neutrophil to lymphocyte ratio in predicting long-term mortality in ST segment elevation myocardial infarction. Am J Cardiol. 2008;101:747–52. doi: 10.1016/j.amjcard.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Turkmen K, Erdur FM, Ozcicek F, Ozcicek A, Akbas EM, Ozbicer A, et al. Platelet-to-lymphocyte ratio better predicts inflammation than neutrophil-to-lymphocyte ratio in end-stage renal disease patients. Hemodial Int. 2013;17:391–6. doi: 10.1111/hdi.12040. [DOI] [PubMed] [Google Scholar]
- 18.Kao SC, Vardy J, Harvie R, Chatfield M, van Zandwijk N, Clarke S, et al. Health-related quality of life and inflammatory markers in malignant pleural mesothelioma. Support Care Cancer. 2013;21:697–705. doi: 10.1007/s00520-012-1569-6. [DOI] [PubMed] [Google Scholar]
- 19.Pinato DJ, Mauri FA, Ramakrishnan R, Wahab L, Lloyd T, Sharma R. Inflammation-based prognostic ýndices in malignant pleural mesothelioma. J Thorac Oncol. 2012;7:587–94. doi: 10.1097/JTO.0b013e31823f45c1. [DOI] [PubMed] [Google Scholar]


