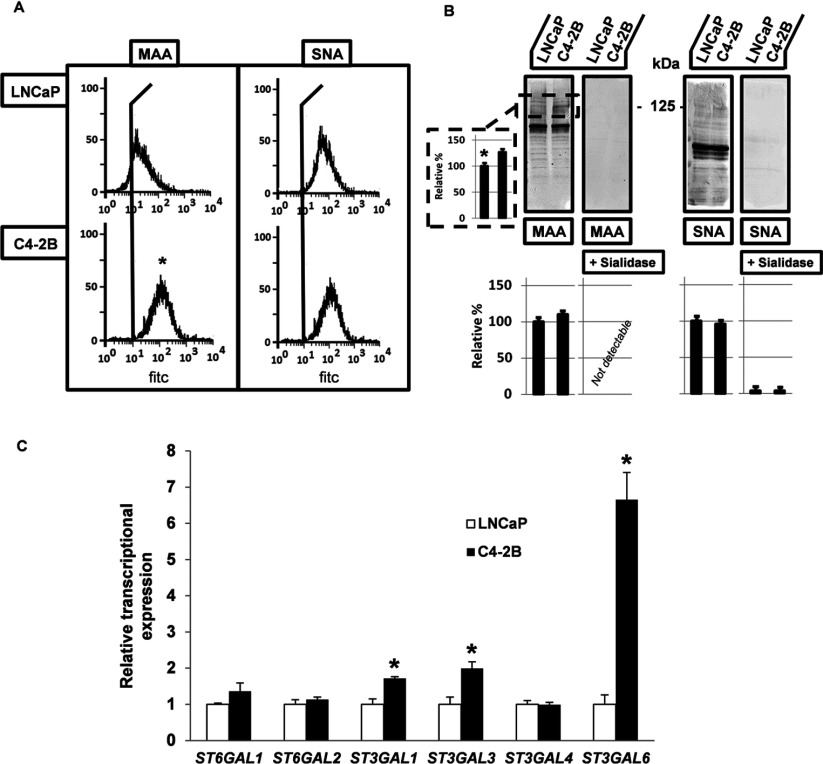
Figure 1. Differences in sialylation between parental, non-invasive LNCaP and bone metastatic C4-2B cells.
(A) Flow cytometric determination of increased cell surface expression levels of α2,3- (left panel) and equal expression levels of α2,6- (right panel) linked sialic acid residues in C4-2B cells, using the lectins MAA and SNA, respectively. Single cell suspensions of LNCaP and C4-2B cells were incubated with biotinylated-MAA and SNA, followed by Fluorescein Avidin DCS and analysed on the Cell Lab Quanta SC MPL, stainings without the particular lectins were used as controls. Each experiment was performed at least three times. (B) Lectin blot analysis for total expression levels of α2,3- and α2,6-linked sialic acids in LNCaP and C4-2B cell lysates, containing 25 μg protein. Lysates were analysed by 4–20% gradient or 7.5% SDS/PAGE, and blotted with MAA (left panel) and SNA (right panel). Sialidase treatment (0.5 U/ml sodium citrate buffer at pH 6) of the blotted membranes for 16 h at 37°C, serves as control for the presence of sialic acid residues. Scion Image densitometry analysis of bands indicating the presence of α2,3- and α2,6-linked sialic acids in both cell lines (lower panels) and evaluation of the increased density at 125 kDa in C4-2B cells (side left panel) (C) Transcriptional expression levels of ST genes resulting in α2,3- and α2,6-linked sialic acid residues. Expression levels were determined by QPCR, normalized against HPRT (hypoxanthine–guanine phosphoribosyltransferase) and levels present in C4-2B cells are expressed relative as compared with the expression in the parental, non-invasive LNCaP cells. Analysed and evaluated data are means±S.D. from at least three independent experiments, asterisks indicate statistical difference from parental, non-invasive LNCaP control cells (P<0.05).