SUMMARY
Neurovascular interactions are essential for proper brain function. While the effect of neural activity on cerebral blood flow has been extensively studied, whether neural activity influences vascular patterning remains elusive. Here, we demonstrate that neural activity promotes the formation of vascular networks in the early postnatal mouse barrel cortex. Using a combination of genetics, imaging, and computational tools to allow simultaneous analysis of neuronal and vascular components, we found that vascular density and branching were decreased in the barrel cortex when sensory input was reduced by either a complete deafferentation, a genetic impairment of neurotransmitter release at thalamocortical synapses, or a selective reduction of sensory-related neural activity by whisker plucking. In contrast, enhancement of neural activity by whisker stimulation led to an increase in vascular density and branching. The finding that neural activity is necessary and sufficient to trigger alterations of vascular networks reveals a novel feature of neurovascular interactions.
INTRODUCTION
Proper function of precisely wired neural circuits depends on a close physical and functional relationship with a complex and overlapping network of blood vessels (Lecrux and Hamel, 2011; Zlokovic, 2010). The brain is more dependent than any other organ on a continuous supply of oxygen and nutrients from blood vessels, and high metabolic activity correlates with higher vascular density (Riddle et al., 1993). Nerves, in turn, control blood vessel dilation and contraction, as well as heart rate. To date, neurovascular interactions are best known for their functional matching in hemodynamics, where increased neural activity leads to increased blood flow (Drake and Iadecola, 2007; Hamel, 2006). However, whether neuronal function and/or neuronal cytoarchitecture have any impact on vascular network structure remains elusive.
Vascular patterning is critical for the proper function of the brain, given its high metabolic demand and vulnerability to ischemia. During early embryonic development, common guidance cues and receptors are responsible for the basic hard wiring of both networks (Carmeliet and Tessier-Lavigne, 2005; Gelfand et al., 2009), and it is well known that after birth neural activity continues to fine-tune neural connectivity (Fu and Zuo, 2011; Katz and Shatz, 1996). Similar microvascular remodelling continues into early postnatal stages. However, compared to the wealth of literature on neural plasticity, knowledge about vascular plasticity is still very limited. The concept of activity-induced vascular plasticity was first introduced only twenty years ago by reports in rats correlating angiogenesis to sensorimotor experience (Black et al., 1990; Black et al., 1987). Since then, very few additional studies have investigated vascular plasticity due to the lack of proper tools to simultaneously visualize the three-dimensional (3-D) structure of both neuronal and vascular modules with high resolution, and it has been controversial whether natural/endogenous neural activity has any impact on vascular patterning (Whiteus et al., 2014).
To address whether neural activity influences vascular structure in the brain, we developed an integrative approach combining mouse genetics, high resolution 3-D imaging, and computational image analysis. We chose the mouse barrel cortex as a model system, where thalamocortical axons (TCAs) organize in a somatotopic sensory map in which one whisker is represented by one barrel (Woolsey, 1978). In this brain region, the neuronal cytoarchitecture is subject to a high degree of plasticity during a critical time window, and neural activity can easily be manipulated through the whisker pathway (Harris and Woolsey, 1981; Kleinfeld and Deschenes, 2011; McCasland and Woolsey, 1988; Woolsey and Wann, 1976). This system thus provides a suitable model to test the role of sensory inputs in shaping vascular networks.
We demonstrate that manipulations of sensory inputs result in vascular structural changes, such that local sensory-related neural activity promotes the formation of cerebrovascular networks. Four different paradigms in which large-scale neuronal cytoarchitecture and neural activity are differentially affected in layer IV were performed: 1) whisker lesions, where both neuronal cytoarchitecture and neural activity are abolished; 2) genetic reduction of thalamocortical neurotransmission where the postsynaptic neuronal cytoarchitecture is abolished and neural activity is reduced; 3) whisker plucking which selectively reduces neural activity while maintaining the neuronal cytoarchitecture; and 4) whisker stimulation leading to enhancement of neural activity only. Sensory deprivation and stimulation resulted in opposing effects on the vascular structure, even in absence of neuroarchitectural changes, indicating that neural activity is necessary for vascular patterning and that changes in neural activity are sufficient to affect vascular structure. Moreover, neuroarchitectural changes did not produce additional vascular alterations, further demonstrating the significant role of neural activity in regulating the structure of cerebrovascular networks. These findings definitively link neural activity to vascular network formation and therefore reveal a novel aspect of neurovascular interactions.
RESULTS
A combination of genetics, imaging and computational tools to study neurovascular development and plasticity in the mouse cerebral cortex
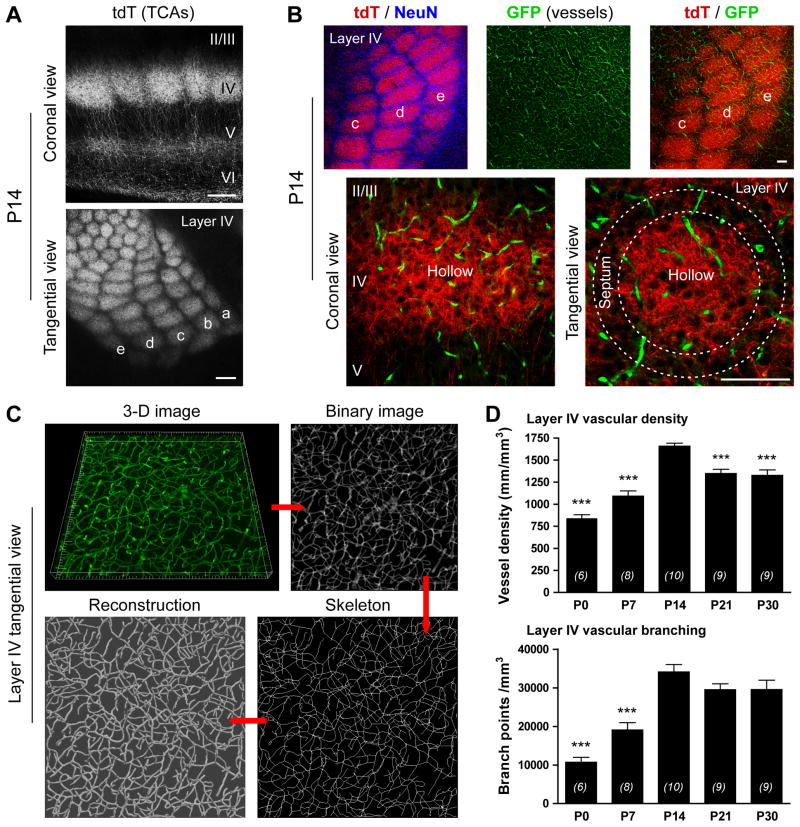
To selectively and simultaneously analyze vascular and neuronal components in layer IV of the barrel cortex, we constructed a compound transgenic mouse in which TCAs are genetically labeled by dTomato (tdT) expression under the serotonin transporter (Sert) promoter (Sert-Cre:tdTflox-stop-flox), and in which blood vessels are labeled through GFP expression under the endothelial-specific Tie2 promoter (Tie2-GFP) (Figures 1A,B and S1A,B). With additional immunostaining for neuronal nuclei (NeuN) revealing cortical neurons that are densely packed and form barrel septa delimiting barrel hollows (TCAs), the neuronal cytoarchitecture of barrel cortex layer IV and its underlying vascular bed are easily visualized with high resolution. As the brain microvasculature develops and elaborates, its complex 3-D structure makes it challenging to detect and quantify fine-scale changes in vascular networks. To overcome this hurdle, we implemented a computational tool which unbiasedly processes 3-D confocal images and automatically quantifies parameters of vascular morphology, including vessel density, vessel diameter, and branching patterns (Figures 1C and S1C; see experimental procedures for details). Moreover, immunostaining with anti-GFP greatly improved the detection of the microvascular bed (Figure S1D). Using this approach, we demonstrate that in layer IV of the barrel cortex vascular density and branching progressively increase after birth and reach a peak at P14 (Figure 1D). Then, from P14 to P30, vascular density and branching decrease (Figure 1D). The values obtained with our image analysis, including vessel density and diameter, are in line with recent studies in the mouse cerebral cortex (Ben-Zvi et al., 2014; Blinder et al., 2013; Harb et al., 2013). The combination of these tools allowed us to ask the fundamental question of whether changes in neuronal cytoarchitecture and/or neural activity lead to structural changes of the brain vasculature.
Figure 1. A combination of genetic, imaging and computational tools to characterize vascular density and branching in layer IV of the postnatal mouse barrel cortex.
A, Thalamocortical axons (TCAs) in layer IV of the barrel cortex as labeled by tdTomato (tdT) expression driven by the serotonin transporter (Sert) promoter. B, A combination of three transgenic mouse lines (Sert-Cre, tdTflox-stop-flox, and Tie2-GFP) allows for simultaneous visualization of TCAs (red) and brain vessels (green). Cortical neurons (neuronal nuclei NeuN immunostaining, blue in upper panel) are organized in barrel septa (outlined by dotted circles in lower panel). C, Overview of the image processing method used to analyze vascular structure (see Fig. S3). D, Vascular density and branching in layer IV of the barrel cortex assayed between P0 and P30. Data are mean ± SEM. Numbers of animals are given in brackets. ***P < 0.001 (vs. P14), one-way ANOVA and Newman-Keuls post-hoc test. Scale bars: 250 μm in A, 100 μm in B.
Developmental profile of neuronal and vascular organization in barrel cortex layer IV
We first examined neurovascular modules in layer IV of the barrel cortex during an early postnatal stage when neural plasticity is in a critical period. At birth (P0), TCAs are only starting to invade the cortex and a rudimentary vasculature is already present in the cortical area where future barrels will form (Figure 2). Between postnatal day 3 (P3) and P5, the vasculature continues to expand while barrel septa (cortical neurons) begin to organize and barrel hollows (TCAs) are hardly noticeable. At P7, barrel hollows and septa become clear and the vasculature has further expanded (Figure 2).
Figure 2. Early postnatal development (P0 to P7) of neural and vascular modules in the mouse barrel cortex.
Coronal view of GFP-expressing vessels (green), tdT-expressing TCAs (red), and NeuN-immunostained cortical neurons (blue). TCAs (arrowheads) start to invade the cortex around birth and clustering of TCAs and cortical neurons into barrel hollows (asterisks) and barrel septa (arrows), respectively, becomes clear at P7. To improve detection, sections were stained by anti-GFP and anti-tdT antibodies. Scale bars: 100 μm.
A complete deafferentation by whisker follicle lesions abolishes the neuroarchitecture and results in a reduction of vascular density and branching in layer IV of the barrel cortex
Since during a critical developmental window (P0 to P5) neuronal circuits undergo massive alteration when neural activity is suppressed (Erzurumlu and Gaspar, 2012; Harris and Woolsey, 1981; Woolsey and Wann, 1976), we hypothesize that neuronal cytoarchitecture and/or neural activity may contribute to the expansion of vascular networks during early life. To test this hypothesis, we first examined the impact of a complete deafferentation on the vasculature in barrel cortex layer IV. When the central row (row c) of whisker follicles is unilaterally lesioned at birth, formation of its cortical representation is impaired, appearsing shrunken at P14, with absence of axonal and neuronal patterning and expansion of surrounding rows (Figure 3A–D and S2A,B). Importantly, analysis of vascular images (Figure S2C,D) revealed a significant reduction of vascular density and branching in layer IV within the contralateral row c compared to the ipsilateral (control) row c (Figure 3D,E). In that volume, the total neuronal density (Figure 3B) and the local neuronal density around vessels (Figure 3C) remained unchanged.
Figure 3. A complete deafferentation by whisker follicle lesions abolishes the neuroarchitecture and results in a reduction of vascular density and branching in layer IV of the barrel cortex.
A–C Analysis of neuronal parameters in barrel row c following whisker row c lesion. A, Total area occupied by TCA clusters in each barrel row. B, Neuronal density within total row c volume. C, Relationship between neuronal density and distance from vessels in row c. No statistical difference was measured (P > 0.05, one-way ANOVA and Newman-Keuls post-hoc test). D, Effect of single (middle panels) or triple (right panels) whisker row lesion on neural and vascular structure in layer IV of the barrel cortex, in the ipsilateral (control) and contralateral (deprived) row c from the same animal. In layer IV of the control (ispsilateral) hemisphere, TCAs and cortical neurons are organized into distinct rows. When whisker follicles are unilaterally cauterized (caut.) at birth, formation of their cortical representation is impaired (absence of axonal and neuronal patterning, and expansion of surrounding rows). Field of view of vascular images in lower panels is outlined by a dotted square in upper left panel. Red brackets delimit the control row c. Red arrowheads point at the deafferented row c. E,F, Quantification of changes in layer IV vascular density and branching following single (E) and triple (F) whiskers row lesion compared to the control hemisphere. Data are mean ± SEM. Numbers of animals are given in brackets. *P < 0.05, **P < 0.01, ***P < 0.001, paired t-test. Scale bars: 200 μm.
We then tested whether inflicting a wider lesion by cauterizing three central whisker rows (b,c and d) could lead to more devastating changes in vascular structure. In this condition, the layer IV neuronal cytoarchitecture appears severely altered in the deafferented row c ‘ghost’ volume (Figure 3D). The microvascular bed within that volume also displayed reduced vascular density and branching (Figure 3F), however no additional reduction (% of decrease) in these parameters was evidenced compared to a single row lesion (P > 0.05, unpaired t-test; Figure 3E,F). Together, these results demonstrate that neuronal cytoarchitecture and/or neural activity promote local formation of vascular networks during the critical period of neural plasticity.
Genetic reduction of neurotransmitter release at thalamocortical synapses impairs postsynaptic neuroarchitecture and leads to a reduction of vascular density and branching in layer IV of the barrel cortex
We next sought to dissect the individual contribution of neuronal cytoarchitecture (TCAs, cortical neurons) versus sensory-related neural activity to vascular plasticity. Presynaptic Rab3-interacting molecules (RIMs) are active zone molecules involved in the control of neurotransmitter release (Kaeser et al., 2011; Wang et al., 1997). Previous work demonstrated that post-synaptic layer IV barrels do not form when neurotransmission is reduced selectively at thalamocortical synapses following presynaptic ablation of both RIM1 and RIM2 in double knockout (RIM DKOSert) mice (Narboux-Neme et al., 2012), as confirmed here (Figure 4A,B and S3A). At P14, a significant reduction of vascular density and branching was observed in barrel cortex layer IV of RIM DKOSert mice compared to littermate controls (Figure 4C). This phenotype was not detected in mice lacking only one RIM isoform in TCAs (RIM1 KOSert or RIM2 KOSert) in which layer IV barrel septa are formed correctly (Figure S3B,C). Moreover, there was no difference between RIM DKOSert and WT mice in the distribution of three major capillary categories based upon different diameters (Figure 4C), suggesting a uniform impairment of vascular growth in these mutants. Similar genetic experiments demonstrated previously that removal of RIMs in TCAs resulted in normal presynaptic ultrastructure, but neurotransmitter release was strongly impaired in layer IV where we examined the vasculature (Narboux-Neme et al., 2012). Therefore, these data demonstrate that reduction of neurotransmitter release at thalamocortical synapses results in both postsynaptic neuronal disorganization and decrease of vascular density and branching in layer IV of the cerebral cortex.
Figure 4. Reduction in neurotransmitter release by genetic ablation of RIMs at thalamocortical synapses results in postsynaptic disorganization and reduction of vascular network formation in layer IV of the barrel cortex.
A, Immunohistochemical stainings at P14 of TCAs with a serotonin transporter (SERT) antibody and of cortical neurons with a neuronal nuclei (NeuN) antibody (white arrows pointing at septa) from tangential sections in layer IV from RIM DKOSert mice and their littermate controls. Dotted white squares indicate the field of view illustrated in C for vascular marker platelet endothelial cell adhesion molecule (PECAM) immunostaining. B, Quantification of the neuronal phenotype observed in RIM DKOSert compared to WT mice, including neuronal density, TCA clusters area, septal area, as well as distribution of NeuN-immunoreactive neurons (blue) and SERT-immunoreactive TCAs (red) across layer IV barrels c3 and c4 (average fluorescence intensity plots; bin size = 10 μm). C, left, sample images (z-projections) of PECAM-immunostained sections from tangential (upper panels) and coronal (lower panels) points of views. Right, 3-D analysis of vessel density, branching and diameter in layer IV vasculature from WT and RIM DKOSert mice. Data are mean ± SEM. Numbers of animals are given in brackets. *P < 0.05, **P < 0.01, one-way ANOVA (including other genotypes shown in Figure S8C) and Newman-Keuls post-hoc test. Scale bars: 250 μm in A, 100 μm in B.
Reduction of sensory-related neural activity by whisker plucking decreases vascular networks formation in layer IV of the barrel cortex
We then aimed to further dissect the contribution of neural activity to vascular network formation. Whisker plucking is a convenient procedure to reduce sensory-related activity in layer IV without inflicting large-scale neuroarchitectural changes (Durham and Woolsey, 1978). Therefore, we performed early whisker plucking during the critical period for barrel cortex plasticity (P0 to P5) (Figures 5 and S2A). Vascular density and branching in the deprived hemisphere exhibited a significant reduction at P14 compared to the ipsilateral hemisphere, while neuronal distribution and density in layer IV were maintained in the deprived hemisphere (Figures 5A-C and S4A). We then plucked the whiskers after the P0-P5 critical period, between P14 and P21, a period when vascular density and branching normally decrease in WT mice (Figures 5C and S2A). This late plucking procedure led to a further decrease in vascular density and branching in layer IV of the contralateral hemisphere at P21 and P30 compared to the ipsilateral hemisphere (Figure 5C), whereas layer IV cytoarchitecture was preserved in the deprived hemisphere (Figure S4C). The reduction in vascular density and branching was specific to layer IV as it was not evidenced in layer V (Figure S4D). Moreover, in both early and late whisker plucking, no change was detected in the distribution of the three main categories of capillaries with different diameters, suggesting a uniform impairment of vascular networks during sensory deprivation (Figure 5C). Since large-scale organization of neuronal cytoarchitecture was unchanged following whisker plucking, these data together demonstrate that reduction of sensory-related neural activity is sufficient to reduce vascular density and branching in the barrel cortex.
Figure 5. Reduction of sensory-related neural activity by whisker plucking leads to a reduction of vascular network formation in layer IV of the barrel cortex.
A, Illustrations (left) and quantifications (right) of neuron (NeuN, blue) and TCA (tdT, red) distribution across layer IV c3 and c4 barrels (average fluorescence intensity plots; bin size = 10 μm) (see Figure S4A). Data were also pooled for septa (S) and hollows (H). B, Organization of TCAs (tdT, red fluoresence) and cortical vessels (GFP, green fluorescence) following early whisker plucking. C, Early whisker plucking: whiskers are plucked from P0 to P5, followed by analysis of vascular structure at P7 and P14. Late whisker plucking: whiskers are plucked from P14 to P21, followed by analysis of vascular structure at P21 and P30 (see Figure S2A). Histograms are quantifications of changes in vessel density, branching and diameter within barrel cortex layer IV. D, Assessment of proliferation of endothelial cells (ECs) and non-endothelial cells (non-ECs) by EdU incorporation following late whisker plucking over ten days (see Figure S4E). Proliferating endothelial nuclei, that are positive for EdU (red), are indicated by arrows; non-proliferating endothelial nuclei are indicated by arrowheads. Top-left insets are a magnification of the area delimited by dotted squares. Data are mean ± SEM. Numbers of animals are given in brackets. *P < 0.05, **P < 0.01, paired t-test (n.s., not significant). Scale bars: 100 μm in A,B, 20 μm in D.
These results contrast with a recent study that found no reduction in vascular density in the early postnatal murine barrel cortex following whisker plucking (Whiteus et al., 2014). However, we noticed a major methodological difference in the image analysis between this study and ours. All our analyses have been done in 3-D, whereas Whiteus et al. performed their analyses in two dimensions (2-D). To examine whether this methodological difference contributes to the divergent conclusions, we re-analyzed part of our early plucking data set using 2-D methods. Vessel density and branching were quantified on maximal intensity z-projections either from the whole z-stack volume (Figure S4B, middle graphs) or from a fixed number of optical sections (Figure S4B, bottom graphs). Data from this analysis are expressed as vessel length and branch points per mm2 (Figure S4B). In contrast to the 3-D analysis, both 2-D analyses failed to reveal any significant difference in vascular density and branching. The lower sensitivity of 2-D analyses may be explained by a loss of information regarding vessel length in the third z dimension, as well as by the difficulty to count branch points and attribute them to a specific vessel in z-projections.
Finally, to test whether the sensory deprivation-induced reduction of vascular density and branching results from decreased angiogenesis, we assessed endothelial cell proliferation in vivo using 5-Ethynyl-2′-deoxyuridine (EdU) incorporation. Following late whisker plucking, the number of proliferating endothelial cells in the deprived hemisphere was significantly reduced compared to the ipsilateral hemisphere, while the number of proliferating non-endothelial cells was unchanged (Figure 5D and S4E). Since angiogenesis is reduced following whisker plucking, these data demonstrate that sensory-related neural activity controls local vascular network formation in the early postnatal cerebral cortex.
Enhancement of sensory inputs by whisker stimulation leads to an increase in vascular density and branching in layer IV of the barrel cortex
To complement our sensory deprivation experiments, we performed a sensory enhancement paradigm by whisker stimulation (Figures 6 and S5), a procedure widely used to increase neural activity in layer IV of the barrel cortex (Lecrux et al., 2011). Whisker stimulation for one week starting at P14 led to significant increase in vascular density and branching in the contralateral hemisphere compared to the ipsilateral hemisphere at P21 (Figure 6A). Increased neural activation was evidenced by the increased number of c-Fos-positive nuclei in the stimulated barrel cortex (Figures 6B and S5B) while large-scale neuronal cytoarchitecture was maintained (Figure S5C). These results demonstrate that enhancement of sensory inputs to the barrel cortex increases vascular density and branching.
Figure 6. Enhancement of sensory-related neural activity by whisker stimulation leads to an increase in vascular density and branching in layer IV of the barrel cortex.
A, Effect of unilateral whisker stimulation for one week (see also Fig. S2A) on vessel density and branching in layer IV in the ipsilateral (control) and contralateral (stimulated) hemispheres from the same animal. B, Effect of unilateral whisker stimulation on neuronal activation in layer IV, as assessed by c-Fos expression. Data are mean ± SEM. Numbers of animals are given in brackets. *P < 0.05, ***P < 0.001, paired t-test. Scale bars: 100 μm.
Reduction of sensory-related neural activity by whisker plucking has no impact on astrocyte distribution and density in layer IV of the barrel cortex
Since astrocytes have been shown to stimulate angiogenesis in vitro by releasing pro-angiogenic molecules in response to glutamate (Munzenmaier and Harder, 2000; Pozzi et al., 2005; Zhang and Harder, 2002), any impairment in the astroglial population might affect its ability to promote angiogenesis. Therefore, we examined the distribution and density of cortical astrocytes in two sensory deprivation paradigms (triple whisker row lesion and whisker plucking). Cerebral cortex astrocytes were labeled for aldehyde dehydrogenase 1 family member L1 (ALDH1L1), an astrocyte-specific marker with pan-astrocyte expression patterns (Cahoy et al., 2008; Molofsky et al., 2012). ALDH1L1-positive protoplasmic astrocytes were found throughout the brain parenchyma and enriched in layers II-III and IV of the cortex, with typical tubular arrangements corresponding to the lining of blood vessels by their fine processes or ‘endfeet’ (Figure 7A,B and S6). Astrocytes appeared accumulated within barrel hollows (Figure 7C,D) and in close contact with both TCAs and microvessels (Figure 7B). GFAP-positive fibrous astrocytes were found almost exclusively in the white matter, as previously described (Cahoy et al., 2008) (Figure S6). Following triple row lesion, both the distribution and cell density of astrocytes in the deprived hemisphere appeared significantly impaired compared to the ipsilateral hemisphere (Figure 7C). In contrast, no detectable differences in astrocyte distribution and cell density were found following whisker plucking, a paradigm which also left the neuronal cytoarchitecture intact. These data demonstrate that large-scale organization of the astroglial population was unchanged following whisker plucking. However, it is still possible that astrocytes function to mediate the effects of neural activity on vascular network formation.
Figure 7. The distribution and cell density of barrel cortex astrocytes are affected by whisker lesion, but not by whisker plucking.
A, Distribution of protoplasmic astrocytes as revealed by ALDH1L1 immunostaining. Low and high magnifications are provided in upper and lower panels, respectively. Arrows designate tubular arrangements of astrocytic processes (endfeets). B, Double (upper panels) and triple (lower panels) immunostainings showing ALDH1L1-positive astrocytes (blue) in close contact with vessels (green) and TCAs (red). Arrows designate astrocytic endfeet lining blood vessels. Examples of close encounter between astrocytes, vessels and nerves are indicated by arrowheads in lower right panel. C,D, Effect of triple whisker row lesion (C) and late whisker plucking (D) on the cellular distribution (average fluorescence intensity plots, bin size of 10 μm, middle panels) and density (right histograms) of barrel cortex astrocytes in layer IV. Data are mean ± SEM. Numbers of animals are given in brackets. **P < 0.01, paired t-test. Scale bars: 50 μm in A, 25 μm in B, 100 μm in C,D. Neuron
DISCUSSION
Our ability to manipulate sensory-related neural activity and to simultaneously examine both neuronal and vascular modules at the same location in barrel cortex layer IV enabled us to demonstrate that ‘natural’ neural activity is necessary for vascular patterning and that changes in neural activity are sufficient to trigger alterations in vascular networks. The opposing impact of sensory deprivation and stimulation on vascular structure specifically in barrel cortex layer IV further demonstrates that neural activity normally promotes vascular network formation in this somatosensory pathway.
We demonstrate that changes in sensory-related neural activity are sufficient to control vascular plasticity, since vascular patterning was affected in absence of large-scale neuronal and astroglial cytoarchitectural changes. Indeed, the reduction in sensory-driven inputs following both whisker lesion and whisker plucking led to a decrease in vascular network formation. In addition, the reduction in vascular density and branching is significantly higher following whisker plucking than following a wide whisker lesion (P < 0.05, unpaired t-test), suggesting that large-scale neuronal cytoarchitectural changes did not produce additional vascular changes.
How does neural activity change the vascular structure? It is possible that sensory-related neural activity directly affects vascular patterning via the release of glutamate by TCAs, or indirectly through pathways that are activated by synaptic transmission involving cortical interneurons and glial cells. It is well known that pyramidal excitatory neurons, inhibitory interneurons and astrocytes are recruited by the whisker-to-barrel pathway (Lecrux et al., 2011), and that in turn they release vasoactive substances which control vascular tone and cerebral blood flow (Cauli and Hamel, 2010; Drake and Iadecola, 2007). Whether angiogenesis regulators are also released by these neuronal modules upon neural activity changes is yet to be examined. Astrocytes, being in close contact with both neuronal synapses and cerebral microvessels, are well positioned to mediate the effects of neural activity on vascular patterning. Indeed, in addition to their role in the control of blood flow (Attwell et al., 2010; Iadecola and Nedergaard, 2007; Lind et al., 2013) and in the maturation and function of neuronal circuits (Chung et al., 2013; Clarke and Barres, 2013; Eroglu et al., 2009), astrocytes are accurate sensors of neural activity and respond to glutamate by releasing pro-angiogenic lipids (epoxy-eicosa-trienoic acids, or EETs) at least as potent as VEGF (Munzenmaier and Harder, 2000; Potente et al., 2003; Pozzi et al., 2005; Zhang and Harder, 2002). Future studies could investigate the precise mechanisms through which neural activity controls the release of EETs in vivo and their effects on vascular patterning in the brain. Such pro-angiogenic mechanisms would likely play a role in improving the balance between metabolic demand and energy supply (Blinder et al., 2013; Riddle et al., 1993).
We demonstrate by a combination of three paradigms of sensory deprivation together with neural activity enhancement that, under physiological conditions, neural activity normally promotes vascular network formation. In contrast, Whiteus et al. found a severe reduction of angiogenesis in the motor cortex after treadmill exercise (45 min, 3 times daily for 5 days), and in the auditory cortex following persistent and repetitive auditory stimulation (over 10 h daily, from P15 to P25) (Whiteus et al., 2014). These hyperactivation paradigms result in widespread inhibition or stimulation in a variety of brain areas. Thus, it is hard to establish a direct physiological link between local neural activity and changes in vascular patterning using these paradigms.
The finding that local neural activity promotes the formation of vascular networks in the cerebral cortex provides new insights into our understanding of neurovascular interactions and is relevant in the context of blood-oxygen-level-dependent (BOLD) functional imaging. In light of the reduced vascular bed following sensory deprivation, additional vascular parameters should be taken into consideration to improve accuracy of BOLD functional imaging studies in sensory-deprived brain regions (e.g. in blindness or deafness). Indeed, it has recently been demonstrated that blood flow, the basis of BOLD imaging, is not solely controlled by arteriole smooth muscle, but also at the capillary level by pericytes (Hall et al., 2014; Hamilton et al., 2010).
Our results obtained in the early postnatal brain also lead us to hypothesize that sensory stimulation may be beneficial to enhance angiogenesis, a paradigm that could potentially be used to prevent or treat early-life ischemic conditions. Indeed, the brain is vulnerable to ischemia, particularly during critical developmental periods when insults trigger irreversible deficits, leading to syndromes such as cerebral palsy (Reddihough and Collins, 2003). Mouse models of perinatal stroke (Tsuji et al., 2013; Vexler et al., 2006) could be used to test this possibility, and whisker stimulation has been shown to enhance endothelial cell proliferation in the ischemic adult barrel cortex (Li et al., 2011; Whitaker et al., 2007).
Finally, our data suggest that the postnatal maturation of brain vascular networks not only relies on genetic programs, but is also controlled by environmental stimuli. It will be important to examine whether neural activity plays a role in the control of cerebrovascular patterning in the healthy brain during adulthood, when neuronal structural plasticity is present but limited.
EXPERIMENTAL PROCEDURES
Animals
Transgenic mouse lines crossed for simultaneous imaging of TCAs and brain vessels were: Sert-Cre (MMRRC:017260-UCD; mixed FVB/B6/129 genetic background), tdTomatoflox-stop-flox (Jackson laboratory, strain 007905; mixed B6/129 background) and Tie2-GFP (Jackson laboratory, strain 003658; FVB/N background). For thalamic ablation of RIM1 and RIM2 proteins, Rim1flox/flox-Rim2flox/flox mice on a mixed B6/129 background (Kaeser et al., 2011; Kaeser et al., 2008) were crossed with the Sert-Cre line. Sert-Cre+/−;Rim1flox/+-Rim2flox/+ mice which were then bred with Rim1flox/flox-Rim2flox/flox mice to obtain Sert-Cre+/−;Rim1flox/flox-Rim2flox/flox (RIM DKOSert), Sert-Cre+/−;Rim1flox/flox-Rim2flox/+ (RIM1 KOSert), and Sert-Cre+/−;Rim1flox/+-Rim2flox/flox (RIM2 KOSert) mice. Mice were raised in standard cages. All animals were treated according to institutional and NIH guidelines approved by IACUC at Harvard Medical School.
Manipulations of sensory inputs
Whisker lesions
Adapted from previous reports (Datwani et al., 2002; Woolsey and Wann, 1976), newborn mice (P0) were anesthetized by hypothermia and the central row (c) or the rows b, c, and d of large whiskers were cauterized under a surgical microscope using an electrosurgery unit (ART-E1, Bonart Co., Keelung City, Taiwan). Neonates were then slowly warmed up on a heating pad (33 C, 20 min), returned to their mothers and euthanized at P7 or P14. The precision and extent of lesions could be verified in the cortex following immunostainings (see below), and mice with imperfect lesions were excluded from the study.
Early whisker plucking
Newborns were anesthetized as above and all large whiskers were plucked from the right side of the snout by applying a gentle tension to the base of the vibrissae (Brown and Dyck, 2003; Fox, 1992; Kossut, 1985). Care was taken to prevent damage to the whisker follicle. Neonates were slowly warmed up, returned to their mothers and euthanized at P7 or P14. In order to maintain sensory deprivation during the critical period for plasticity (P0-P5), vibrissae were checked for regrowth and replucked if necessary until P5.
Late whisker plucking
P14 animals were anesthetized using isoflurane (3% induction, 1.5%–2% maintenance, in 100% oxygen) and all large whiskers were plucked as above. Mice were returned to their mothers and euthanized at P21 or P30. For assessment of cell proliferation in vivo, all large whiskers were plucked from P14 to P24. Vibrissae were checked for regrowth every other day until sacrifice.
Whisker stimulation
Adapted from previous studies (Filipkowski et al., 2000; Lecrux et al., 2011), pups where first habituated to the experimental conditions and were then subjected to daily whisker stimulation for 8 days (P14 to P21). Each day, pups where gently restrained on the top of a plastic cylinder and their right whiskers were manually stimulated for 15 min (3–4 Hz) using a paint brush. In our case, since whisker plucking led to reduction of vascular network density and branching, the contralateral whiskers were left intact. Sham controls were manipulated but not stimulated. Mice were euthanized at P21, 1 h after the last stimulation.
Immunohistochemistry
For all experiments except in vivo EdU incorporation (see below), mice were euthanized at experimental endpoints by cervical dislocation and the brain was removed from the skull. For a coronal view of cerebral structures, the whole brain was fixed by immersion in 4% paraformaldehyde (PFA) overnight at 4°C. For a tangential view of cortical layers, the cortex was dissected in ice-cold PBS, flattened between two glass slides and fixed by immersion in 4% PFA overnight at 4°C. Fixation by immersion allows all brains from each experimental group to be fixed exactly the same way and at the same time, reducing inter-animal variability. Fixed brains and flatten cortices were then rinsed in PBS, embedded in 2% agarose in PBS, and cut coronally (whole brain) or tangentially (flatten cortex) into serial, thick sections (50 μm for 2-D illustrations; 120 μm for 3-D vascular reconstructions) using a vibratome (Leica VT1000S). Sections were blocked with 10% horse serum, permeabilized with 0.2% Triton X-100, and incubated overnight with one or a mixture of the following primary antibodies: rabbit α-GFP (1:1000; A-11122, Life Technologies), α-DsRed (1:500; 632496, Clontech), α-c-Fos (1:250; sc-52, Santa Cruz Biotechnoloy, Inc), α-ALDH1L1 (1:500; ab87117, Abcam), guinea pig α-NeuN (1:1000; ABN90, EMD Millipore), rat α-PECAM-1 (1:200; 553370, BD Pharmingen™), and goat α-SERT (1:500; sc-1458, Santa Cruz Biotechnoloy, Inc), followed by species-specific 568/488 Alexa Fluor-conjugated (Invitrogen) or Cy™5-conjugated (Jackson ImmunoResesarch) secondary antibodies (1:300). Slides were mounted in Fluoromount G (EMS) and visualized by epifluorescence or confocal microscopy.
In vivo assessment of cell proliferation
Four mice were subjected to a slightly modified ‘late plucking’ paradigm for a ten day deprivation. All large whiskers were plucked from P14 to P24. To assess cell proliferation in vivo, we used the sensitive method of 5-Ethynyl-2′-deoxyuridine (EdU) incorporation. EdU detection only requires fast chemical staining compatible with high-resolution immunohistochemistry (Cappella et al., 2008; Salic and Mitchison, 2008). Adapted from previous reports (Salic and Mitchison, 2008; Zeng et al., 2010), mice received EdU injections (100 μg/day, in PBS, i.p.) from P14 to P23, and were euthanized at P24 by transcardial perfusion with 4% PFA under deep anesthesia. Their brain was post-fixed in 4% PFA, cryoprotected in 30% sucrose and embedded for cryostat sectioning. EdU was detected on 40 μm-thick sections using a Click-iT® Alexa Fluor® 594 EdU Imaging Kit (C10340, Life Technologies) following manufacturer’s protocol. Briefly, slides were washed in 3% bovine serum albumin (BSA) in PBS, permeablized with 0.5% Triton, washed again in 3% BSA-PBS and then incubated with the Click-iT® reaction cocktail. Slides were then washed in 3% BSA-PBS and processed for immunohistochemistry to label vessels (PECAM-1) and nuclei (DAPI). The proportions of proliferating endothelial cells (ECs) and non-endothelial cells (non-ECs) were assessed by manually counting EdU-positive and EdU-negative nuclei in the ipsi- versus contra-lateral hemispheres from confocal images (60x oil immersion objective, 1x zoom, 1 μm optical sections) using ImageJ Cell Counter (n=4, three brain sections per animal, five images per hemisphere on each section).
Image acquisition
Immunostained sections were examined under a laser scanning confocal microscope (Olympus FluoView™ FV1000). For single image illustrations, maximal intensity z-projections were obtained from 5 to 10 μm z-stacks acquired using either a 4x, 10x, 20x or 60x objective (1 μm optical sections, 1x zoom). For 3-D reconstruction of vascular networks in flattened cortex tangential sections, layer IV of the posteromedial barrel field was located using tdT fluorescence under a 10x objective, and then 50 to 70 μm deep z-stacks (1 μm optical sections, 1x zoom) were acquired for the vascular GFP signal in the core of layer IV using a 20x objective (Figure S1C).
For temporal characterization of layer IV vasculature (Figure 1D), adjacent z-stacks covering the width of the barrel field were acquired in the right hemisphere.
For whisker lesion experiments (Figure 3), one z-stack centered over barrel row c was acquired in each brain side: in the control hemisphere (ipsilateral to the lesion) and in the lesioned (contralateral) hemisphere (Figure S2C).
For conditional loss of RIM proteins (Figure 4), 3 adjacent z-stacks covering the width of the barrel field were acquired in the right hemisphere from immunostained sections of mutant and littermates control mice.
For whisker plucking (and stimulation) experiments (Figure 5), 3 adjacent z-stacks covering the width of the barrel field were acquired in the control and manipulated hemispheres (Figure S2D).
Images and illustrations were processed using ImageJ (NIH), Adobe Photoshop CS6 and Adobe Illustrator CS6.
Computational analysis of cortical vasculature
Data collection
For whisker lesion experiments, the volume of the row c in the intact hemisphere, delimited by boundaries between neuronal septa (NeuN), was considered as the internal control (Figures 3D and S2C). In the contralateral hemisphere following single row lesion, layer IV vessels were analyzed within the “lesioned volume” (Figure S2C), whose borders are defined by neuronal septa (NeuN) from rows b and d. Following triple row lesions, layer IV vessels were analyzed within the row c ‘ghost’ volume (Figure S2C), which corresponds to a projection of the control row c volume over the mispatterned field in the deprived hemisphere. Dotted lines in Figures 3D and S2C represents either the anatomical borders of controls and lesioned row c, or border of the projected row c ‘ghost’ volume.
All vascular measurements from raw z-stack files were analyzed by a person blind to experimental conditions.
Computational morphometric analysis of 3-D vascular images
The algorithms used to process the stacks were implemented in Python 2.7, using the following python modules: Numpy, Scipy, Matplotlib, Opencv2, Igraph and Scikit-Image.
Each image was smoothed in order to reduce noise by using the Gradient Anisotropic Diffusion filter from SimpleITK software (http://www.simpleitk.org/). An adaptive thresholding algorithm (Gonzalez and Woods, 2007) with a window size of 100 μm was applied to each image in the z-stack. If the intensity of a pixel was greater than the mean intensity of pixels inside the window centered on the concerned pixel, this pixel was classified as belonging to a vessel. The resulting binary image may contain pixels incorrectly classified as vessel pixels. This was corrected by deleting image components smaller than 500 μm3 (Shapiro and Stockman, 2001). Background pixels may remain inside vessels (i.e., holes). In order to fill such holes, background components smaller than 100 μm3 were removed. The final binary image was obtained by doing a closing operation (Dougherty and Lotufo, 2003) with a 6-pixel-wide disk.
Skeletonization has been used previously for geometric characterization of biological shapes (Cesar and Costa, 1999; Meng et al., 2008; Rafelski et al., 2012; Viana et al., 2009). In the current work, the skeleton of the blood vessels was obtained by a thinning algorithm (Palàgyi and Kuba, 1998) and was then represented as a network. Each pixel having three or more neighbors was classified as a branching point, and pixels having only one neighbor were identified as terminal points. The positions of branching and termination points, as well as all points of the segments, were stored for analysis.
Noise and irregularities in vascular structure may generate some spurious short segments. An iterative algorithm was applied to sequentially remove them. Segments smaller than 10 μm were recurrently erased, starting from the endpoints of the skeletons. The length of each segment was then estimated by using a differential estimate of the arc length (Costa and Cesar Jr., 2009). We also measured the number of branching points in each z-stack. Finally, in order to validate the analysis, we created 3-D images containing both the original image and the final skeletons, and verified that the obtained skeletons were accurately representing the original blood vessel structure.
For vessel radius estimation, each skeleton segment was linearly interpolated and smoothed. Each point of the transformed segment was associated to a plane which is perpendicular to the segment, passing through the point. This plane was used to find the cross section of the binary vessel image at the point. The area of the cross section defines the equivalent radius of the blood vessel at the point trough the formula , which is the radius of a disk of area. We eliminate points that are closer than 3 μm to a termination or branching of the segment. Finally, the median of the radius calculated on the remaining points was associated to the whole segment.
Computational morphometric analysis of 2-D vascular images
Two analyses were done in the early plucking data set (three randomly chosen animals) to quantify vessel density and branching in 2-D. These two analyses differ one another on the number of z-stack planes used to create the 2-D image. In the first one, we used the whole z-stack volume for the 2-D z-projection. In the second, we first extracted a region of interest from the z-stacks. This region was centered in the middle of the z-stack and was 15 μm deep. For both analyses, the respective 2-D image was obtained by doing a maximum intensity z-projection of the extracted region. The algorithm to characterize the 2-D images was similar to that used for 3-D images, the only difference being the binarization procedure, which is described in step 1 of the previous section. For the 2-D analysis, we used a window size of 220 μm for the adaptive thresholding, and components smaller than 100 μm3 were removed.
Estimation of neuronal density and its relationship to vessels in 3-D (Figure 3B,C)
The 3-D NeuN-positive neuronal nuclei images were first smoothed using a Gaussian filter. Then, an adaptive threshold with a window size of 100 μm was applied to each plane of the z-stack. Components smaller than 150 μm3 were removed. Nuclei that were merged into a single component were separated by detecting their respective centers, which was done by using the distance transform (Costa and Cesar Jr., 2009). The result of this procedure is a new image containing the centroid of each neuronal nucleus.
The neuronal density was estimated using the binary blood vessel image and the centroids found in the previous step. The binary image was used as a reference to define shells around the blood vessels, each shell containing all image points with distance larger than x and smaller than x+Δx from the border of the binary blood vessel structure. The neuronal density for the shell was then calculated as the number of neuron centroids inside the shell divided by the shell volume. The width of the shell was set as Δx=2.5 μm (Travencolo et al., 2007).
Cell counting and average intensity plots
Quantifications were adapted from previous reports (Mangin et al., 2012; Narboux-Neme et al., 2012).
Confocal images (20x objective, 1 μm optical sections, 1x zoom) and maximal intensity z-projections (5 optical sections) were used for quantification. Using the Cell Counter plugin of ImageJ (NIH), we obtained the number of neurons by counting NeuN-positive nuclei, and the number of astrocytes by counting the number of ALDH1L1-positive soma colocalizing with DAPI nuclear staining in the region of interest (ROI, e.g. barrel row, barrel hollow, or barrel septa). All quantifications were done on a ROI including row c barrels c3 and c4 which display similar size. The interbarrel ‘septal’ area was defined using tdT-positive TCAs as the total area of ROI minus the sum of tdT-positive clusters. Densities were obtained by dividing the number of cells by the area of the ROI (mean of c3 and c4 barrels for neurons, whole row c and row c ghost for astrocytes).
Average intensity plots were done using the Plot Profile function in ImageJ (NIH). We either show average image profile plots in which intensity value of several adjacent pixels are averaged (10 μm per bin), or average profile plots profiles where values from septa and hollows are pooled.
Statistical methods
Vascular morphological features obtained blindly from the computational analysis were regrouped and averaged for each animal to obtain an individual value (score). Scores obtained for each parameter for each animal were then averaged within experimental groups. Statistical analyses were performed using Prism4 (GraphPad Software). Multiple groups comparisons were analyzed using a one-way ANOVA followed by a Newman-Keuls post-hoc test. Two groups comparisons were analyzed using paired or unpaired Student’s t-test. Data reported are mean ± SEM. P < 0.05 was considered statistically significant.
Supplementary Material
Figure S1 (Related to main Figure 1). An integrated approach to study the development and plasticity of neurovascular modules in the mouse cerebral cortex. A,B, Generation of a compound transgenic mouse to simultaneously analyze neural and vascular structure in the mouse cerebral cortex. A, Compound mouse generation strategy (Sert-Cre:tdTomatoflox-stop-flox;Tie2-GFP) in which thalamocortical axons (TCAs) are labeled by tdTomato (tdT) expression after Cre recombination under the control of the serotonin transporter (Sert) promoter. Brain vessels are labeled by GFP expression under the endothelial Tie2 promoter. B, tdT expression in compound mice reveals the entire somatosensory map in layer IV of the barrel cortex. Anti-tdT immunostaining was used to enhance and stabilize fluorescence. a1, primary auditory cortex; fl, forelimb; Fr, frontal cortex; hl, hindlimb; ll, lower lip; lw, large whiskers; sw, small whiskers; t, trunk. C, Computational image analysis to unbiasedly and automatically extract vascular structural features (see experimental procedures for details) such as vessel density, diameter and branching. In brief, each image pixel is classified as belonging to a vessel or to the image background. A series of filters and algorithms is then applied to obtain the skeleton of the vessels, providing a concise description of the overall network. This skeleton is used to obtain the topology of the blood vessels’ volumetric representation. D, The use of anti-GFP immunostaining greatly improves the detection of the microvascular bed. E, Analysis of vascular density in septum versus hollow of layer IV barrels. The septum/hollow ratio for vascular density was calculated and appeared almost equal to one, with no statistical difference between the two subregions. Scale bars: 750 μm in B, 100 μm in E.
Figure S2 (Related to main Figures 3, 5 and 6). Experimental design, controls and analyses. A, Experimental designs for manipulation of sensory-related neural activity. For whisker follicle lesions, selected follicles were cauterized at birth and vascular structure was analyzed at P7 and P14. For whisker plucking experiments, all whiskers were plucked unilaterally, either during the critical period for plasticity (P0-P5, ‘early plucking’) or during the late period when vascular density and branching decrease (P14-P21, ‘late plucking’) and vascular structure was analyzed at P21 and P30. For whisker stimulation experiments, all whiskers were stimulated unilaterally between P14 and P21 and the vasculature analyzed at P21. For all vascular structural analysis, tissue was collected at the indicated time points. See experimental procedures for details. B, Histological controls at P14 showing the consequence of whisker lesions at birth. Left, Section from the mystacial pad skin showing absence of row c follicles (between arrowheads) in the lesioned side. Right, Tangential brain sections in layer IV showing the effect of a triple row lesion on neuronal (NeuN) cytoarchitecture. C, Illustration of image analysis for whisker cautery experiments. Central row (row c) volume from the intact hemisphere (left), delimited by boundaries between barrel septa (dotted line), was considered as the internal control and used as a reference volume to analyze vessels locally (yellow rectangle between dotted lines, lower panel). Following row c cautery (middle), layer IV vessels are analyzed within the ‘lesioned volume’. Following triple row cautery (right), vessels are analyzed within the row c ‘ghost’ volume which corresponds to a projection of the control volume over the mispatterned field in the deprived hemisphere. See experimental procedures for details. D, For whisker plucking experiments, adjacent z-stacks covering the width of the cortical representation of large whiskers are acquired in the control (ipsilateral) and deprived (contralateral) hemispheres. Values are obtained for each z-stack, averaged for each hemisphere, and means per animal are then pooled in experimental groups. Scale bars: 1 mm in B (left), 200 μm in B (right), 100 μm in D.
Figure S3 (Related to main Figure 4). Mice lacking only RIM1 or RIM2 in TCAs display normal barrel cortex with normal vasculature. A,B, In both RIM1 KOSert and RIM2 KOSert mice, respectively lacking RIM1 or RIM2 in TCAs, double-immunostaining for SERT (TCAs) and NeuN (neuronal nuclei) did not reveal any overt abnormality in the organization of neuronal modules, as illustrated on coronal views. B,C, Morphometric analysis of vascular density and branching did not reveal any alteration in RIM1 KOSert and RIM2 KOSert mice. The field of view of the PECAM staining images is indicated by dotted squares in B. Numbers of animals are given in brackets. Scale bars: 250 μm in A, 100 μm in B.
Figure S4 (Related to main Figure 5). Further analysis of neuronal and vascular parameters following whisker plucking. A, Analysis of neuronal parameters in the early whisker plucking paradigm. Left and middle, Distribution of average fluorescence intensity (normalized, septal values being 1) of neurons (NeuN, blue) and TCAs (tdT, red) across layer IV barrels c3 and c4 (profile plots, data pooled for septum, S, and hollow, H). Right, Analysis of neuronal (NeuN) mean density ratio (septum/hollow) in layer IV barrels c3 and c4. B, Comparison between 3-D and 2-D image analysis in 3 randomly chosen animals from the early plucking data set. Quantifications of vessel density and branching were done either on whole volume 3-D reconstructions (upper graphs), on z-projections from the whole volume (middle graphs), or from a fixed number of optical sections (lower graphs). See Results and Experimental procedures for details. C, Late whisker plucking results in reduction of vascular growth while large-scale neuroarchitecture is unchanged, as illustrated at P30. D, Analysis of vascular density and branching in barrel cortex layer V following early whisker plucking. E, Experimental design (left) and supplemental images (right) from in vivo assessment of cell proliferation by EdU incorporation. Arrows in upper panels designate a proliferating endothelial cell (EC) identified by its EdU/DAPI-positive nucleus. Arrowheads in lower panels designate non-ECs also found proliferating in the cortical parenchyma. Data are mean ± S.E.M. Numbers of animals are given in brackets. Scale bars: 100 μm in B (left), 250 μm in B (right), 10 μm in E.
Figure S5 (Related to main Figure 6). Whisker stimulation leads to enhanced c-Fos expression in barrel cortex layer IV as well as increased vascular complexity. A, No difference in vascular structure was observed in sham-manipulated (unstimulated) animals (right versus left hemisphere). B, c-Fos immunoreactivity is almost exclusively detected in the stimulated hemisphere. C, The overall barrel structure of is not affected by whisker stimulation. Numbers of animals are given in brackets. Scale bar: 100 μm.
Figure S6 (Related to main Figure 7). Distribution of astrocytes in selected regions of the mouse forebrain. Immunostaining for GFAP (left) or ALDH1L1 (right) in the barrel cortex (upper panels), dorsal hippocampus (middle panels) and dorsolateral striatum (bottom panels). Arrows designate tubular arrangements of astrocytic endfeet. CA1, CA1 pyramidal layer of the hippocampus; cg, cingulum; DG, dendate gyrus; ec, external capsule; CPu, caudate putamen. The distribution of ALDH1L1 mean fluorescence intensity across cortical layers is given in the top right graph. Scale bars: 150 μm.
HIGHLITGHTS.
Neural activity promotes the formation of vascular networks in the cerebral cortex.
Sensory deprivation reduces vascular complexity in layer IV of the barrel cortex.
Genetic impairment of neurotransmitter release impairs cortical vascular structure.
Activity induced vascular changes occur even in absence of neuroarchitectural changes.
Acknowledgments
We thank Drs. Christopher Harvey and Jonathan Cohen, and members of the Gu laboratory, for constructive comments on the manuscript; Dr. Susan Dymecki for providing the Slc6a4-Cre mouse; Drs. Lisa Goodrich and Bernardo Sabatini for sharing their lab equipment; the Neurobiology Imaging Facility in the department of Neurobiology at Harvard Medical School (facility supported in part by the Neural Imaging Center as part of an NINDS P30 Core Center grant #NS072030) and the Enhanced Neuroimaging Core at Harvard NeuroDiscovery Center for helping with confocal imaging; Lydia Bickford for technical assistance. This work was supported by the Mahoney postdoctoral fellowship (B.L.), the Goldenson and Lefler postdoctoral fellowships (A.B.Z), by FAPESP grant 2011/22639-8 (C.H.C.), FAPESP grant 11/50761-2, CNPQ grant 304351/2009-1 (L.d.F.C.),.), grant K01DA029044 from NIH/NIDA (P.S.K.), and by the following grants to C.G.: Harvard/MIT Joint Research Grants Programm in Basic Neuroscience, Sloan research fellowship, the Genise Goldenson fund, the Freudenberger award, and NIH grant R01NS064583.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zvi A, Lacoste B, Kur E, Andreone BJ, Mayshar Y, Yan H, Gu C. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature. 2014;509:507–511. doi: 10.1038/nature13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci U S A. 1990;87:5568–5572. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JE, Sirevaag AM, Greenough WT. Complex experience promotes capillary formation in young rat visual cortex. Neurosci Lett. 1987;83:351–355. doi: 10.1016/0304-3940(87)90113-3. [DOI] [PubMed] [Google Scholar]
- Blinder P, Tsai PS, Kaufhold JP, Knutsen PM, Suhl H, Kleinfeld D. The cortical angiome: an interconnected vascular network with noncolumnar patterns of blood flow. Nat Neurosci. 2013;16:889–897. doi: 10.1038/nn.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CE, Dyck RH. Experience-dependent regulation of synaptic zinc is impaired in the cortex of aged mice. Neuroscience. 2003;119:795–801. doi: 10.1016/s0306-4522(03)00292-6. [DOI] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappella P, Gasparri F, Pulici M, Moll J. A novel method based on click chemistry, which overcomes limitations of cell cycle analysis by classical determination of BrdU incorporation, allowing multiplex antibody staining. Cytometry Part A: the journal of the International Society for Analytical Cytology. 2008;73:626–636. doi: 10.1002/cyto.a.20582. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Tessier-Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436:193–200. doi: 10.1038/nature03875. [DOI] [PubMed] [Google Scholar]
- Cauli B, Hamel E. Revisiting the role of neurons in neurovascular coupling. Front Neuroenergetics. 2010;2:9. doi: 10.3389/fnene.2010.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesar RM, Jr, Costa LD. Computer-vision-based extraction of neural dendrograms. J Neurosci Methods. 1999;93:121–131. doi: 10.1016/s0165-0270(99)00120-x. [DOI] [PubMed] [Google Scholar]
- Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, Joung J, Foo LC, Thompson A, Chen C, et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013;504:394–400. doi: 10.1038/nature12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke LE, Barres BA. Emerging roles of astrocytes in neural circuit development. Nat Rev Neurosci. 2013;14:311–321. doi: 10.1038/nrn3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LdF, Cesar RM., Jr . Shape classification and analysis: theory and practice. 2. CRC Press; Boca Raton, Florida: 2009. [Google Scholar]
- Datwani A, Iwasato T, Itohara S, Erzurumlu RS. Lesion-induced thalamocortical axonal plasticity in the S1 cortex is independent of NMDA receptor function in excitatory cortical neurons. J Neurosci. 2002;22:9171–9175. doi: 10.1523/JNEUROSCI.22-21-09171.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty ER, Lotufo RA. Hands-on Morphological Image Processing. 1. SPIE Publications; Bellingham, Washington: 2003. [Google Scholar]
- Drake CT, Iadecola C. The role of neuronal signaling in controlling cerebral blood flow. Brain Lang. 2007;102:141–152. doi: 10.1016/j.bandl.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Durham D, Woolsey TA. Acute whisker removal reduces neuronal activity in barrels of mouse SmL cortex. J Comp Neurol. 1978;178:629–644. doi: 10.1002/cne.901780403. [DOI] [PubMed] [Google Scholar]
- Eroglu C, Allen NJ, Susman MW, O’Rourke NA, Park CY, Ozkan E, Chakraborty C, Mulinyawe SB, Annis DS, Huberman AD, et al. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139:380–392. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzurumlu RS, Gaspar P. Development and critical period plasticity of the barrel cortex. Eur J Neurosci. 2012;35:1540–1553. doi: 10.1111/j.1460-9568.2012.08075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipkowski RK, Rydz M, Berdel B, Morys J, Kaczmarek L. Tactile experience induces c-fos expression in rat barrel cortex. Learn Mem. 2000;7:116–122. doi: 10.1101/lm.7.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K. A critical period for experience-dependent synaptic plasticity in rat barrel cortex. J Neurosci. 1992;12:1826–1838. doi: 10.1523/JNEUROSCI.12-05-01826.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M, Zuo Y. Experience-dependent structural plasticity in the cortex. Trends Neurosci. 2011;34:177–187. doi: 10.1016/j.tins.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand MV, Hong S, Gu C. Guidance from above: common cues direct distinct signaling outcomes in vascular and neural patterning. Trends in cell biology. 2009;19:99–110. doi: 10.1016/j.tcb.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez RC, Woods RE. Digital Image Processing. 3. Prentice Hall; New Jersey: 2007. [Google Scholar]
- Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, O’Farrell FM, Buchan AM, Lauritzen M, Attwell D. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508:55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol (1985) 2006;100:1059–1064. doi: 10.1152/japplphysiol.00954.2005. [DOI] [PubMed] [Google Scholar]
- Hamilton NB, Attwell D, Hall CN. Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front Neuroenergetics. 2010:2. doi: 10.3389/fnene.2010.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harb R, Whiteus C, Freitas C, Grutzendler J. In vivo imaging of cerebral microvascular plasticity from birth to death. J Cereb Blood Flow Metab. 2013;33:146–156. doi: 10.1038/jcbfm.2012.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RM, Woolsey TA. Dendritic plasticity in mouse barrel cortex following postnatal vibrissa follicle damage. J Comp Neurol. 1981;196:357–376. doi: 10.1002/cne.901960302. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- Kaeser PS, Deng L, Wang Y, Dulubova I, Liu X, Rizo J, Sudhof TC. RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ-domain interaction. Cell. 2011;144:282–295. doi: 10.1016/j.cell.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeser PS, Kwon HB, Chiu CQ, Deng L, Castillo PE, Sudhof TC. RIM1alpha and RIM1beta are synthesized from distinct promoters of the RIM1 gene to mediate differential but overlapping synaptic functions. J Neurosci. 2008;28:13435–13447. doi: 10.1523/JNEUROSCI.3235-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kleinfeld D, Deschenes M. Neuronal basis for object location in the vibrissa scanning sensorimotor system. Neuron. 2011;72:455–468. doi: 10.1016/j.neuron.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossut M. Effects of sensory denervation and deprivation on a single cortical vibrissal column studied with 2-deoxyglucose. Physiologia Bohemoslovaca. 1985;34(Suppl):79–83. [PubMed] [Google Scholar]
- Lecrux C, Hamel E. The neurovascular unit in brain function and disease. Acta Physiol (Oxf) 2011;203:47–59. doi: 10.1111/j.1748-1716.2011.02256.x. [DOI] [PubMed] [Google Scholar]
- Lecrux C, Toussay X, Kocharyan A, Fernandes P, Neupane S, Levesque M, Plaisier F, Shmuel A, Cauli B, Hamel E. Pyramidal neurons are “neurogenic hubs” in the neurovascular coupling response to whisker stimulation. J Neurosci. 2011;31:9836–9847. doi: 10.1523/JNEUROSCI.4943-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WL, Fraser JL, Yu SP, Zhu J, Jiang YJ, Wei L. The role of VEGF/VEGFR2 signaling in peripheral stimulation-induced cerebral neurovascular regeneration after ischemic stroke in mice. Exp Brain Res. 2011;214:503–513. doi: 10.1007/s00221-011-2849-y. [DOI] [PubMed] [Google Scholar]
- Lind BL, Brazhe AR, Jessen SB, Tan FC, Lauritzen MJ. Rapid stimulus-evoked astrocyte Ca2+ elevations and hemodynamic responses in mouse somatosensory cortex in vivo. Proc Natl Acad Sci U S A. 2013;110:E4678–4687. doi: 10.1073/pnas.1310065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangin JM, Li P, Scafidi J, Gallo V. Experience-dependent regulation of NG2 progenitors in the developing barrel cortex. Nat Neurosci. 2012;15:1192–1194. doi: 10.1038/nn.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCasland JS, Woolsey TA. High-resolution 2-deoxyglucose mapping of functional cortical columns in mouse barrel cortex. J Comp Neurol. 1988;278:555–569. doi: 10.1002/cne.902780407. [DOI] [PubMed] [Google Scholar]
- Meng S, Costa LdF, Geyer SH, Viana MP, Reiter C, Muller GB, Weninger WJ. Three-dimensional description and mathematical characterization of the parasellar internal carotid artery in human infants. Journal of Anatomy. 2008;212:636–644. doi: 10.1111/j.1469-7580.2008.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, Krencik R, Ullian EM, Tsai HH, Deneen B, Richardson WD, Barres BA, Rowitch DH. Astrocytes and disease: a neurodevelopmental perspective. Genes Dev. 2012;26:891–907. doi: 10.1101/gad.188326.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munzenmaier DH, Harder DR. Cerebral microvascular endothelial cell tube formation: role of astrocytic epoxyeicosatrienoic acid release. Am J Physiol Heart Circ Physiol. 2000;278:H1163–1167. doi: 10.1152/ajpheart.2000.278.4.H1163. [DOI] [PubMed] [Google Scholar]
- Narboux-Neme N, Evrard A, Ferezou I, Erzurumlu RS, Kaeser PS, Laine J, Rossier J, Ropert N, Sudhof TC, Gaspar P. Neurotransmitter release at the thalamocortical synapse instructs barrel formation but not axon patterning in the somatosensory cortex. J Neurosci. 2012;32:6183–6196. doi: 10.1523/JNEUROSCI.0343-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palàgyi K, Kuba A. A 3D 6-subiteration thinning algorithm for extracting medial lines. Pattern Recognition Letters. 1998;19:613–627. [Google Scholar]
- Potente M, Fisslthaler B, Busse R, Fleming I. 11,12-Epoxyeicosatrienoic acid-induced inhibition of FOXO factors promotes endothelial proliferation by down-regulating p27Kip1. J Biol Chem. 2003;278:29619–29625. doi: 10.1074/jbc.M305385200. [DOI] [PubMed] [Google Scholar]
- Pozzi A, Macias-Perez I, Abair T, Wei S, Su Y, Zent R, Falck JR, Capdevila JH. Characterization of 5,6- and 8,9-epoxyeicosatrienoic acids (5,6- and 8,9-EET) as potent in vivo angiogenic lipids. J Biol Chem. 2005;280:27138–27146. doi: 10.1074/jbc.M501730200. [DOI] [PubMed] [Google Scholar]
- Rafelski SM, Viana MP, Zhang Y, Chan YH, Thorn KS, Yam P, Fung JC, Li H, Costa LdF, Marshall WF. Mitochondrial network size scaling in budding yeast. Science. 2012;338:822–824. doi: 10.1126/science.1225720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddihough DS, Collins KJ. The epidemiology and causes of cerebral palsy. The Australian journal of physiotherapy. 2003;49:7–12. doi: 10.1016/s0004-9514(14)60183-5. [DOI] [PubMed] [Google Scholar]
- Riddle DR, Gutierrez G, Zheng D, White LE, Richards A, Purves D. Differential metabolic and electrical activity in the somatic sensory cortex of juvenile and adult rats. J Neurosci. 1993;13:4193–4213. doi: 10.1523/JNEUROSCI.13-10-04193.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salic A, Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci U S A. 2008;105:2415–2420. doi: 10.1073/pnas.0712168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro LG, Stockman GC. Computer Vision. Prentice Hall; New Jersey: 2001. [Google Scholar]
- Travencolo BA, Martinez Debat C, Beletti ME, Sotelo Silveira JR, Ehrlich R, da Costa LF. A new method for quantifying three-dimensional interactions between biological structures. J Anat. 2007;210:221–231. doi: 10.1111/j.1469-7580.2006.00685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji M, Ohshima M, Taguchi A, Kasahara Y, Ikeda T, Matsuyama T. A novel reproducible model of neonatal stroke in mice: comparison with a hypoxia-ischemia model. Exp Neurol. 2013;247:218–225. doi: 10.1016/j.expneurol.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Vexler ZS, Sharp FR, Feuerstein GZ, Ashwal S, Thoresen M, Yager JY, Ferriero DM. Translational stroke research in the developing brain. Pediatric neurology. 2006;34:459–463. doi: 10.1016/j.pediatrneurol.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Viana MP, Tanck E, Beletti ME, Costa LdF. Modularity and robustness of bone networks. Molecular bioSystems. 2009;5:255–261. doi: 10.1039/b814188f. [DOI] [PubMed] [Google Scholar]
- Wang Y, Okamoto M, Schmitz F, Hofmann K, Sudhof TC. Rim is a putative Rab3 effector in regulating synaptic-vesicle fusion. Nature. 1997;388:593–598. doi: 10.1038/41580. [DOI] [PubMed] [Google Scholar]
- Whitaker VR, Cui L, Miller S, Yu SP, Wei L. Whisker stimulation enhances angiogenesis in the barrel cortex following focal ischemia in mice. J Cereb Blood Flow Metab. 2007;27:57–68. doi: 10.1038/sj.jcbfm.9600318. [DOI] [PubMed] [Google Scholar]
- Whiteus C, Freitas C, Grutzendler J. Perturbed neural activity disrupts cerebral angiogenesis during a postnatal critical period. Nature. 2014;505:407–411. doi: 10.1038/nature12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey TA. Some anatomical bases of cortical somatotopic organization. Brain, behavior and evolution. 1978;15:325–371. doi: 10.1159/000123786. [DOI] [PubMed] [Google Scholar]
- Woolsey TA, Wann JR. Areal changes in mouse cortical barrels following vibrissal damage at different postnatal ages. J Comp Neurol. 1976;170:53–66. doi: 10.1002/cne.901700105. [DOI] [PubMed] [Google Scholar]
- Zeng C, Pan F, Jones LA, Lim MM, Griffin EA, Sheline YI, Mintun MA, Holtzman DM, Mach RH. Evaluation of 5-ethynyl-2′-deoxyuridine staining as a sensitive and reliable method for studying cell proliferation in the adult nervous system. Brain Res. 2010;1319:21–32. doi: 10.1016/j.brainres.2009.12.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Harder DR. Cerebral capillary endothelial cell mitogenesis and morphogenesis induced by astrocytic epoxyeicosatrienoic Acid. Stroke. 2002;33:2957–2964. doi: 10.1161/01.str.0000037787.07479.9a. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. Neurodegeneration and the neurovascular unit. Nat Med. 2010;16:1370–1371. doi: 10.1038/nm1210-1370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 (Related to main Figure 1). An integrated approach to study the development and plasticity of neurovascular modules in the mouse cerebral cortex. A,B, Generation of a compound transgenic mouse to simultaneously analyze neural and vascular structure in the mouse cerebral cortex. A, Compound mouse generation strategy (Sert-Cre:tdTomatoflox-stop-flox;Tie2-GFP) in which thalamocortical axons (TCAs) are labeled by tdTomato (tdT) expression after Cre recombination under the control of the serotonin transporter (Sert) promoter. Brain vessels are labeled by GFP expression under the endothelial Tie2 promoter. B, tdT expression in compound mice reveals the entire somatosensory map in layer IV of the barrel cortex. Anti-tdT immunostaining was used to enhance and stabilize fluorescence. a1, primary auditory cortex; fl, forelimb; Fr, frontal cortex; hl, hindlimb; ll, lower lip; lw, large whiskers; sw, small whiskers; t, trunk. C, Computational image analysis to unbiasedly and automatically extract vascular structural features (see experimental procedures for details) such as vessel density, diameter and branching. In brief, each image pixel is classified as belonging to a vessel or to the image background. A series of filters and algorithms is then applied to obtain the skeleton of the vessels, providing a concise description of the overall network. This skeleton is used to obtain the topology of the blood vessels’ volumetric representation. D, The use of anti-GFP immunostaining greatly improves the detection of the microvascular bed. E, Analysis of vascular density in septum versus hollow of layer IV barrels. The septum/hollow ratio for vascular density was calculated and appeared almost equal to one, with no statistical difference between the two subregions. Scale bars: 750 μm in B, 100 μm in E.
Figure S2 (Related to main Figures 3, 5 and 6). Experimental design, controls and analyses. A, Experimental designs for manipulation of sensory-related neural activity. For whisker follicle lesions, selected follicles were cauterized at birth and vascular structure was analyzed at P7 and P14. For whisker plucking experiments, all whiskers were plucked unilaterally, either during the critical period for plasticity (P0-P5, ‘early plucking’) or during the late period when vascular density and branching decrease (P14-P21, ‘late plucking’) and vascular structure was analyzed at P21 and P30. For whisker stimulation experiments, all whiskers were stimulated unilaterally between P14 and P21 and the vasculature analyzed at P21. For all vascular structural analysis, tissue was collected at the indicated time points. See experimental procedures for details. B, Histological controls at P14 showing the consequence of whisker lesions at birth. Left, Section from the mystacial pad skin showing absence of row c follicles (between arrowheads) in the lesioned side. Right, Tangential brain sections in layer IV showing the effect of a triple row lesion on neuronal (NeuN) cytoarchitecture. C, Illustration of image analysis for whisker cautery experiments. Central row (row c) volume from the intact hemisphere (left), delimited by boundaries between barrel septa (dotted line), was considered as the internal control and used as a reference volume to analyze vessels locally (yellow rectangle between dotted lines, lower panel). Following row c cautery (middle), layer IV vessels are analyzed within the ‘lesioned volume’. Following triple row cautery (right), vessels are analyzed within the row c ‘ghost’ volume which corresponds to a projection of the control volume over the mispatterned field in the deprived hemisphere. See experimental procedures for details. D, For whisker plucking experiments, adjacent z-stacks covering the width of the cortical representation of large whiskers are acquired in the control (ipsilateral) and deprived (contralateral) hemispheres. Values are obtained for each z-stack, averaged for each hemisphere, and means per animal are then pooled in experimental groups. Scale bars: 1 mm in B (left), 200 μm in B (right), 100 μm in D.
Figure S3 (Related to main Figure 4). Mice lacking only RIM1 or RIM2 in TCAs display normal barrel cortex with normal vasculature. A,B, In both RIM1 KOSert and RIM2 KOSert mice, respectively lacking RIM1 or RIM2 in TCAs, double-immunostaining for SERT (TCAs) and NeuN (neuronal nuclei) did not reveal any overt abnormality in the organization of neuronal modules, as illustrated on coronal views. B,C, Morphometric analysis of vascular density and branching did not reveal any alteration in RIM1 KOSert and RIM2 KOSert mice. The field of view of the PECAM staining images is indicated by dotted squares in B. Numbers of animals are given in brackets. Scale bars: 250 μm in A, 100 μm in B.
Figure S4 (Related to main Figure 5). Further analysis of neuronal and vascular parameters following whisker plucking. A, Analysis of neuronal parameters in the early whisker plucking paradigm. Left and middle, Distribution of average fluorescence intensity (normalized, septal values being 1) of neurons (NeuN, blue) and TCAs (tdT, red) across layer IV barrels c3 and c4 (profile plots, data pooled for septum, S, and hollow, H). Right, Analysis of neuronal (NeuN) mean density ratio (septum/hollow) in layer IV barrels c3 and c4. B, Comparison between 3-D and 2-D image analysis in 3 randomly chosen animals from the early plucking data set. Quantifications of vessel density and branching were done either on whole volume 3-D reconstructions (upper graphs), on z-projections from the whole volume (middle graphs), or from a fixed number of optical sections (lower graphs). See Results and Experimental procedures for details. C, Late whisker plucking results in reduction of vascular growth while large-scale neuroarchitecture is unchanged, as illustrated at P30. D, Analysis of vascular density and branching in barrel cortex layer V following early whisker plucking. E, Experimental design (left) and supplemental images (right) from in vivo assessment of cell proliferation by EdU incorporation. Arrows in upper panels designate a proliferating endothelial cell (EC) identified by its EdU/DAPI-positive nucleus. Arrowheads in lower panels designate non-ECs also found proliferating in the cortical parenchyma. Data are mean ± S.E.M. Numbers of animals are given in brackets. Scale bars: 100 μm in B (left), 250 μm in B (right), 10 μm in E.
Figure S5 (Related to main Figure 6). Whisker stimulation leads to enhanced c-Fos expression in barrel cortex layer IV as well as increased vascular complexity. A, No difference in vascular structure was observed in sham-manipulated (unstimulated) animals (right versus left hemisphere). B, c-Fos immunoreactivity is almost exclusively detected in the stimulated hemisphere. C, The overall barrel structure of is not affected by whisker stimulation. Numbers of animals are given in brackets. Scale bar: 100 μm.
Figure S6 (Related to main Figure 7). Distribution of astrocytes in selected regions of the mouse forebrain. Immunostaining for GFAP (left) or ALDH1L1 (right) in the barrel cortex (upper panels), dorsal hippocampus (middle panels) and dorsolateral striatum (bottom panels). Arrows designate tubular arrangements of astrocytic endfeet. CA1, CA1 pyramidal layer of the hippocampus; cg, cingulum; DG, dendate gyrus; ec, external capsule; CPu, caudate putamen. The distribution of ALDH1L1 mean fluorescence intensity across cortical layers is given in the top right graph. Scale bars: 150 μm.