Abstract
Purpose
The purpose of this study is to investigate the association of cigarette smoking with gastric cancer.
Methods
Over 215,000 men and women, representing five ethnic groups (African Americans, Japanese Americans, Latino Americans, Native Hawaiians, and Whites), completed a mailed questionnaire, 1993–1996. After an average follow-up of 7.3 years, 454 men and 242 women were diagnosed with gastric adenocarcinoma. Cox proportional hazard models were used to calculate multivariate-adjusted hazard ratios and 95% confidence intervals.
Results
Current cigarette smokers had elevated hazard ratios compared with never smokers among men (HR = 1.98; 95% CI 1.46–2.70) and women (HR = 1.78; 95% CI 1.23–2.57). This positive association was consistent across all five ethnicities. Former smokers had an elevated risk among men, but not among women. There was a significant trend by intensity (cigarettes per day) and duration (years) of smoking among all current smokers. After separation by anatomic location of their tumor, ever smokers had a higher risk for gastric cardia cancer (HR = 2.86; 95% CI 1.66–4.93) than for distal gastric cancer (HR = 1.52; 95% CI 1.25–1.86) among men and women combined. Analysis by histologic tumor type showed a stronger association between current smoking and the intestinal type.
Conclusions
Overall, this study shows an association of current cigarette smoking with gastric cancer in both sexes, consistency of this effect across five ethnic groups, evidence for a dose–response effect of smoking in both sexes, a stronger effect for cardia than for distal gastric cancer, and a stronger association for intestinal than for diffuse gastric cancer.
Keywords: Gastric cancer, Cohort study, Cigarette smoking
Introduction
Although the incidence of gastric cancer is declining in many western countries, it is still the second most common cause of cancer deaths in the world [1, 2]. A recent metaanalysis of cohort studies concluded that smoking should be classified as the most important behavioral risk factor for gastric cancer [3]. However, there are still issues that need to be resolved. There is the possibility of a differential effect by sex with men experiencing a higher gastric cancer risk due to cigarette smoking [3]. The evidence of a dose–response effect has not been consistent [3–5]. The association of cigarette smoking with the anatomical sub-site of the stomach needs to be investigated further, especially since there has been a decline in the incidence of cancer of the distal stomach, but an increase in the occurrence of gastric cardia cancer in the United States [6]. In addition, little information is available on the effect of cigarette smoking by histological subtypes of gastric cancer [3]. For these reasons, we decided to do an analysis of a large cohort study to examine the association between cigarette smoking and gastric cancer risk in multiethnic populations in Hawaii and California. Although the past cohort studies have been done in Europe, Asia, and the United States [3], none have reported on the association across multiple ethnic groups in the same study population.
Materials and methods
Study design and population
The Multiethnic Cohort (MEC) Study in Hawaii and Los Angeles was designed to investigate the association of dietary, lifestyle, and genetic factors with the incidence of cancer and other chronic diseases. Its study design, questionnaire development, subject recruitment, and data collection have been described elsewhere [7]. Briefly, over 215,000 men and women, age 45–75, living in Hawaii or in California (mainly in Los Angeles County) completed a 26-page self-administered mailed questionnaire between 1993 and 1996. The primary sampling frame for the study was the drivers’ license files in both states, because they included the names of most adult residents, contained information on age, and encompassed all socio-economic strata.
Study participants provided information on their diet (including alcohol intake), body weight and height, demographic factors (including education), lifestyle practices (including smoking history), history of medical conditions, use of medications (including aspirin), and a family history of common cancers. The following questions were asked regarding cigarette smoking history: (1) Have you ever smoked a total of 20 or more packs of cigarettes in your lifetime? (2) If yes, what is the total number of years you smoked? (3) What is the average number of cigarettes that you smoked per day; and (4) If you quit smoking, how long ago did you quit? All questionnaire data were checked for consistency and legibility prior to scanning and stored in a secured database. The institutional review boards at the University of Hawaii and at the University of Southern California approved the study protocol.
Study exclusion criteria
For this analysis, we limited the study participants to the five major ethnic groups recruited into the study (African Americans, Japanese Americans, Latinos, Native Hawaiians, and Whites). Latinos were defined as persons of Mexican or South or Central American ancestry, including immigrants from those countries. We excluded relatively small numbers of Chinese, Filipinos, and members of other ethnic groups (n = 13,994). We also excluded 8,264 persons with invalid dietary data and 558 subjects with a gastric cancer diagnosis before baseline that was either self-reported in the questionnaire or identified by registry linkages. In addition, 7,499 participants who provided an incomplete cigarette smoking history were removed. As a result, 185,506 participants remained in the study.
Surveillance
Since 1993, the cohort has been under surveillance for gastric cancer incidence by record linkage to the Hawaii Tumor Registry, the Cancer Surveillance Program for Los Angeles County and the California State Cancer Registry, and for mortality by record linkage to the death certificate files in Hawaii and California and to the National Death Index. All three cancer registries are members of the NCI’s Surveillance, Epidemiology and End Results (SEER) Program [8]. The out-migration rate in the cohort has been low at 3.7% after 7 years of follow-up; therefore, few cases should be missed through passive follow-up. Case ascertainment was complete through December 31, 2004. The International Classification of Diseases (ICD)−03 coding, used by the registries, identified the stomach cancer cases (code C16). The cases in this study were limited to patients diagnosed with invasive adenocarcinoma of the stomach (715 cases). Gastric cancer patients, who had a gastric cancer other than adenocarcinoma (n = 75) or who were diagnosed with carcinoma-in situ (n = 17), were not included as cases.
The following codes specified the tumor location: C16.0 Cardia, not otherwise specified (NOS); C16.1 Fundus; C16.2 Body of stomach; C16.3 gastric antrum; C16.4 pylorus; C16.5 lesser curvature of stomach, NOS; C16.6 greater curvature of stomach, NOS; C16.8 overlapping lesion of stomach; C16.9 stomach, NOS. Code 16.0 identified gastric cardia cases and codes 16.1–16.8 identified distal gastric cancer cases. A tumor was coded 16.8 (overlapping lesion of stomach) when the location of the lesion was in the anterior or posterior wall of the stomach and none of the specific sites was mentioned (cardia, fundus, body of stomach, etc.) in the description of the tumor. Cases with code 16.9 (stomach, not otherwise specified) were cases with missing site codes, and they were not included in the site-specific analysis in the paper.
A limited number of cases were coded according to the histologic classification of Lauren [9]. In the SEER site/ histology codes, code 8144 identified intestinal cases, code 8145 identified diffuse cases, and code 8255 identified mixed type of adenocarcinoma cases. Cases with code 8255 were not included in the analysis by histologic type.
Statistical analysis
We applied Cox proportional hazards models using age as the time metric to calculate hazard ratios and 95% CI. Follow-up began at the date of cohort entry, defined as questionnaire completion or, for the few individuals (n = 1,113) who were slightly younger than 45 years of age when they completed the baseline questionnaire, as the date the participant turned 45. Follow-up ended at the earliest of the following dates: date of gastric cancer diagnosis, date of death, or December 31, 2004, the closure date for this analysis. The other gastric cancer cases that were not included as events were censored at the time of their diagnosis. It was important to model smoking exposure correctly. Therefore, we developed a comprehensive model where the relationship between smoking variables and stomach cancer incidence was allowed to vary over time, based on the model developed to study tobacco use and lung cancer in the MEC [10]. However, the results were similar between the time-dependent and baseline value only models; therefore, the more parsimonious model is shown. Tests of the proportional hazards assumptions for the exposure and adjustment variables based on Schoenfeld residuals showed no violations for any analysis [11].
The following adjustment factors were used in the multivariate model: age at cohort entry, ethnicity as a strata variable, family history of gastric cancer (yes/no), education (less than high school/high school or more), aspirin use (any regular use/never regular use), processed meat intake in terms of density (continuous variable, in g/kcal/day), alcohol consumption (continuous variable, in g/day), and body mass index (BMI) (continuous variable, weight in kg divided by the square of height in meters). In the adjustment for ethnicity, Japanese American and Latino American individuals were separated into groups based on place of birth due to the association of foreign-born birthplace and gastric cancer incidence (which is potentially reflective of Helicobacter pylori prevalence) in our population. As a result, the ethnicity groups were categorized as follows: White, African American, Native Hawaiian, Japanese American (first generation), Japanese American (second generation), Japanese American (third generation or later), Latino American (first generation), and Latino American (second generation or later). Subjects with missing data for any of the adjustment variables were excluded. Consequently, 182,441 participants were included in the multivariate analysis. Cigarette-years was computed as duration of smoking in years times the average number of cigarettes smoked per day, with never smokers assigned 0 [12]. Exposure variables were generally categorized and represented in the models as indicator variables. Trend tests were conducted by inclusion of a continuous variable in the model assigned the median values of the appropriate intensity or duration category. Heterogeneity by sex and ethnicity was tested based on the Wald test of cross-product terms. Heterogeneity by cancer subsite was tested using competing risks methodology [11]. When the analyses were repeated in a sensitivity analysis excluding all individuals with prevalent cancer of any type at baseline, the results were similar.
All analyses were performed using SAS Statistical Software, version 9 (SAS Institute Inc., Cary, NC), and all statistical tests were two-sided.
Results
There were 82,683 men and 99,758 women included in this study. The mean duration of follow-up was 7.3 years, with a total of over 1.4 million person-years of observation. During that time, 454 men and 242 women were diagnosed with gastric cancer. The mean age at diagnosis was 68.5 for men and 68.0 for women.
Table 1 shows the baseline characteristics of gastric cancer cases and the total study population by sex. The cases were older at time of study entry and more likely to have a family history of gastric cancer. They were also less educated and less likely to have a history of aspirin use.
Table 1.
Baseline characteristics of gastric cancer cases and the study population by sex
| Baseline characteristics | Men |
Women |
||
|---|---|---|---|---|
| Cases (n = 454) | Population (n = 82,683) |
Cases (n = 242) | Population (n = 99,758) |
|
| Age at study entry (years) | 65.8 ± 7.4 | 60.1 ± 8.9 | 65.3 ± 7.1 | 59.6 ± 8.8 |
| Race-ethnicity (%) | ||||
| African American | 10.6 ± 1.4 | 13.3 ± 0.1 | 18.6 ± 2.5 | 19.3 ± 0.1 |
| Japanese American | 53.5 ± 2.3 | 30.3 ± 0.2 | 43.8 ± 3.2 | 27.7 ± 0.1 |
| Latino | 18.9 ± 1.8 | 23.5 ± 0.1 | 20.2 ± 2.6 | 20.6 ± 0.1 |
| Native Hawaiian | 6.8 ± 1.2 | 7.1 ± 0.1 | 7.9 ± 1.7 | 7.5 ± 0.1 |
| White | 10.1 ± 1.4 | 25.8 ± 0.2 | 9.5 ± 1.9 | 24.9 ± 0.1 |
| Cigarette smoking status (%) | ||||
| Never smoker | 19.4 ± 1.9 | 30.8 ± 0.2 | 61.2 ± 3.1 | 56.6 ± 0.2 |
| Former smoker | 62.1 ± 2.3 | 51.1 ± 0.2 | 22.3 ± 2.7 | 29.0 ± 0.1 |
| Current smoker | 18.5 ± 1.8 | 18.1 ± 0.1 | 16.5 ± 2.4 | 14.4 ± 0.1 |
| High school education (%) | 43.6 ± 2.3 | 59.1 ± 0.2 | 35.5 ± 3.1 | 54.6 ± 0.2 |
| Family history at gastric cancer (%) | 11.0 ± 1.5 | 4.9 ± 0.1 | 12.4 ± 2.1 | 5.1 ± 0.1 |
| Aspirin user (%) | 35.7 ± 2.2 | 39.9 ± 0.2 | 26.0 ± 2.8 | 35.6 ± 0.2 |
| BMI (kg/m2) | 25.2 ± 3.6 | 26.1 ± 4.1 | 25.9 ± 6.0 | 26.0 ± 5.7 |
| Alcohol intake (g/day) | 13.5 ± 29.0 | 14.7 ± 32.5 | 2.5 ± 8.8 | 4.3 ± 15.0 |
| Processed meat intake (g 1,000 kcal−1 day−1) | 9.0 ± 6.7 | 8.9 ± 7.2 | 6.7 ± 5.4 | 6.9 ± 6.2 |
All values are x ± SD or % ± SE
Table 2 presents the hazard ratios for gastric cancer by smoking status and sex. Men had higher hazard ratios among current cigarette smokers (HR = 1.98; 95% CI 1.46–2.70) and former cigarette smokers (HR = 1.74; 95% CI 1.37–2.22) compared with never smokers. For women, a higher hazard ratio was present among current smokers (HR = 1.78), but not among former smokers (HR = 0.94). The effect for current smoking was similar between men and women (p for interaction = 0.44), while the effect for former smoking was significantly different (p = 0.0025).
Table 2.
Adjusted hazard ratios and 95% CI for gastric cancer by cigarette smoking status and sex
| Cases | Hazard ratio | 95% CI | |
|---|---|---|---|
| Men (n = 82,683) | 454 | ||
| Never smoker | 88 | 1.00 | |
| Ever smoker | 366 | 1.79 | 1.41–2.26 |
| Former smoker | 282 | 1.74 | 1.37–2.22 |
| Current smoker | 84 | 1.98 | 1.46–2.70 |
| Women (n = 99,758) | 242 | ||
| Never smoker | 148 | 1.00 | |
| Ever smoker | 94 | 1.16 | 0.88–1.52 |
| Former smoker | 54 | 0.94 | 0.68–1.29 |
| Current smoker | 40 | 1.78 | 1.23–2.57 |
| Total (n = 182,441)a | 696 | ||
| Never smoker | 236 | 1.00 | |
| Ever smoker | 460 | 1.48 | 1.25–1.76 |
| Former smoker | 336 | 1.40 | 1.16–1.67 |
| Current smoker | 124 | 1.79 | 1.42–2.25 |
Adjusted for age at cohort entry as a continuous variable, ethnicity as a strata variable, education, processed meat intake, BMI, alcohol intake, aspirin use, and family history of gastric cancer
Additional adjustment for sex
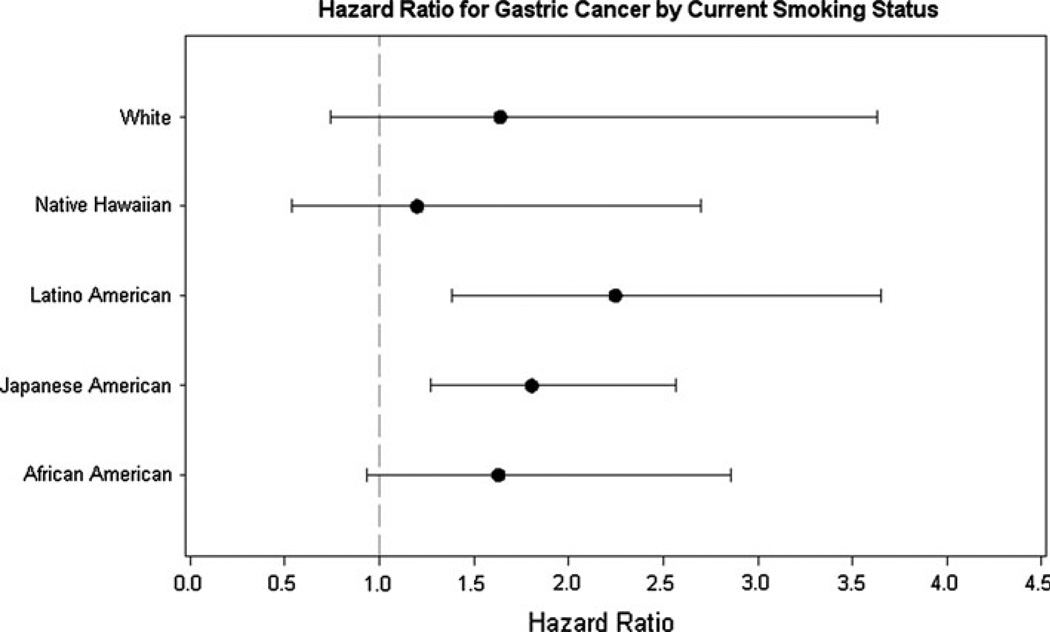
To examine the consistency of the association between current cigarette smoking and gastric cancer, the hazard ratios were determined for each of the five ethnic groups. The eight ethnic-generational groups were collapsed here, because the numbers in the generational groups were too small for estimation. Men and women were combined in the analysis, because of the relatively small numbers of cases when they were separated into sex-ethnic-specific groupings. The results are shown in Fig. 1. The hazard ratios were greater than one for all five groups and significant for Japanese and Latino Americans. The differences were not significant across ethnic groups (p for interaction = 0.87).
Fig. 1.
The hazard ratios and 95% CI of current smokers compared with never smokers for gastric cancer are shown for the five ethnic groups among men and women combined. The hazard ratios were estimated from cox regression with age as the time metric and adjusted for age, family history of gastric cancer, education, aspirin use, processed meat intake, alcohol consumption, and BMI
Among former male smokers, there was a linear trend in risk, based on years since quitting smoking cigarettes, as shown in Table 3. After adjustment for cigarette-years, those who quit for 21 or more years had a 17% excess risk compared with nonsmokers, while those who quit within the past 10 years had a 69% excess risk. There was no trend among women (data not shown), as female former smokers did not have an elevated risk compared with nonsmokers.
Table 3.
Adjusted hazard ratios for gastric cancer by smoking status and years since quitting smoking cigarettes among men
| Cases (n = 454) | Hazard ratio | 95% CI | |
|---|---|---|---|
| Never smoker | 88 | 1.00 | |
| Former smoker: time since quitting (years) | |||
| 21+ | 97 | 1.17 | 0.83–1.66 |
| 11–20 | 85 | 1.42 | 0.93–2.19 |
| ≤10 | 100 | 1.69 | 1.04–2.76 |
| p for trend | 0.024 | ||
| Current smoker | 84 | 1.98 | 1.46–2.70 |
Adjusted for age at cohort entry as a continuous variable, ethnicity, education, processed meat intake, BMI, alcohol intake, aspirin use, cigarette-years, and family history of gastric cancer
Table 4 presents the dose–response relationship for gastric cancer by number of cigarettes smoked per day and years of smoking among current smokers. The analysis for cigarettes per day was not adjusted for duration of smoking, because of the high correlation between these two variables (r = 0.89 for all, 0.78 for men, and 0.95 for women) and inclusion of both variables severely attenuated both effects. Those who smoked 21 or more cigarettes per day had the highest hazard ratio among men (HR = 2.15), women (HR = 2.15), and both sexes combined (HR = 2.16).
Table 4.
Adjusted hazard ratios for gastric cancer by intensity and duration of current cigarette smoking
| Smoking intensity | Cases | Hazard ratio | 95% CI |
|---|---|---|---|
| Men (n = 40,453) | 172 | ||
| Never smoker | 88 | 1.00 | |
| Current smoker (cigarettes/day) | |||
| ≤10 | 27 | 1.89 | 1.20–2.95 |
| 11–20 | 33 | 1.76 | 1.16–2.67 |
| 21+ | 24 | 2.15 | 1.34–3.48 |
| p for trend | 0.0004 | ||
| Women (n = 70,855) | 188 | ||
| Never smoker | 148 | 1.00 | |
| Current smoker (cigarettes/day) | |||
| ≤10 | 22 | 1.88 | 1.18–2.99 |
| 11–20 | 12 | 1.50 | 0.82–2.75 |
| 21+ | 6 | 2.15 | 0.92–5.01 |
| p for trend | 0.0166 | ||
| Total (n = 111,308)a | 360 | ||
| Never smoker | 236 | 1.00 | |
| Current smoker (cigarettes/day) | |||
| ≤10 | 49 | 1.87 | 1.36–2.58 |
| 11–20 | 45 | 1.68 | 1.19–2.35 |
| 21+ | 30 | 2.16 | 1.43–3.26 |
| p for trend | <0.0001 | ||
| Smoking duration | |||
| Men (n = 40,453) | 172 | ||
| Never smoker | 88 | 1.00 | |
| Current smoker (years) | |||
| ≤30 | 23 | 1.97 | 1.21–3.21 |
| 31–40 | 23 | 2.14 | 1.32–3.49 |
| 41+ | 38 | 1.75 | 1.17–2.61 |
| p for trend | 0.0005 | ||
| Women (n = 70,855) | 188 | ||
| Never smoker | 148 | 1.00 | |
| Current smoker (years) | |||
| ≤30 | 14 | 1.51 | 0.85–2.67 |
| 31–40 | 10 | 1.59 | 0.82–3.07 |
| 41+ | 16 | 2.29 | 1.34–3.92 |
| p for trend | 0.0014 | ||
| Total (n = 111,308)a | 360 | ||
| Never smoker | 236 | 1.00 | |
| Current smoker (years) | |||
| ≤30 | 37 | 1.74 | 1.20–2.51 |
| 31–40 | 33 | 1.91 | 1.30–2.81 |
| 41+ | 54 | 1.89 | 1.37–2.60 |
| p for trend | <0.0001 | ||
Adjusted for age at cohort entry as a continuous variable, ethnicity, education, processed meat intake, BMI, alcohol intake, aspirin use, and family history of gastric cancer
Former smokers are not included in this analysis
Additional adjustment for sex
There was a significant trend in the hazard ratios by duration of smoking among current smokers for men (p <0.0005) and women (p = 0.0014), but those who smoked for the longest duration did not have the highest ratios among men. Rather, it appeared that the risk plateaued after duration of 31 years or more when men and women were combined. The difference in effect of duration in men and women was not significant (p for interaction = 0.56).
Table 5 separated the cases according to the anatomical location of the cancer in the stomach: cardia and non-cardia or distal. The location of the tumor in 68 cases was not specified. There were 104 cases of cardia cancer (87 men and 17 women), which constituted just 15% of the total number of gastric cancer cases in the study. For cardia cancer, there was a positive association for ever smokers (includes current and former smokers) among men (HR = 2.60; 95% CI 1.41–4.80) and women (HR = 4.11; 95% CI 1.37–12.30). For distal gastric cancer, the association with ever smokers existed only for men (HR = 1.82; 95% CI 1.38–2.41). When former and current smokers were considered separately, significant hazard ratios for cardia cancer were present for both male and female former smokers, in spite of the low numbers of cases among women. When men and women were combined, the hazard ratios for cardia cancer were higher than those for distal gastric cancer, and this difference was significant.
Table 5.
Adjusted hazard ratios for gastric cancer by anatomic site, cigarette smoking status, and sex
| Gastric cardia cancer |
Distal gastric cancer |
p value for interaction |
|||||
|---|---|---|---|---|---|---|---|
| Cases | Hazard ratio | 95% CI | Cases | Hazard ratio | 95% CI | ||
| Men (n = 82,683) | 87 | 322 | |||||
| Never smoker | 12 | 1.00 | 62 | 1.00 | |||
| Ever smoker | 75 | 2.60 | 1.41–4.80 | 260 | 1.82 | 1.38–2.41 | 0.0015 |
| Former smoker | 52 | 2.28 | 1.21–4.29 | 205 | 1.81 | 1.36–2.41 | 0.0077 |
| Current smoker | 23 | 3.97 | 1.94–8.12 | 60 | 1.85 | 1.28–2.69 | 0.0001 |
| Women (n = 99,758) | 17 | 202 | |||||
| Never smoker | 5 | 1.00 | 122 | 1.00 | |||
| Ever smoker | 12 | 4.11 | 1.37–12.30 | 80 | 1.25 | 0.93–1.68 | 0.0048 |
| Former smoker | 9 | 4.29 | 1.38–13.35 | 45 | 0.98 | 0.60–1.40 | 0.0054 |
| Current smoker | 3 | 3.58 | 0.79–16.24 | 35 | 1.99 | 1.34–2.97 | 0.0543 |
| Total (n = 182,441)a | 104 | 524 | |||||
| Never smoker | 17 | 1.00 | 184 | 1.00 | |||
| Ever smoker | 87 | 2.86 | 1.66–4.93 | 340 | 1.52 | 1.25–1.86 | <0.0001 |
| Former smoker | 61 | 2.56 | 1.46–4.49 | 250 | 1.44 | 1.17–1.78 | <0.0001 |
| Current smoker | 26 | 4.02 | 2.11–7.63 | 90 | 1.80 | 1.38–2.36 | <0.0001 |
Adjusted for age at cohort entry as a continuous variable, ethnicity, education, processed meat intake, BMI, alcohol intake, aspirin use, and family history of gastric cancer
Additional adjustment for sex
Of the 696 gastric cancer patients in the study, only a limited number (258) had been coded by the histologic classification of Lauren into intestinal type (183) and diffuse type (75), men and women combined. Compared with never smokers, current smokers had a hazard ratio of 2.06 (95% CI 1.30–3.26) for the intestinal type and 1.18 (95% CI 0.52–2.65) for the diffuse type of gastric cancer; these hazard ratios are significantly different (p = 0.03).
Discussion
Among current cigarette smokers in our MEC study, the risk of gastric adenocarcinoma was increased in both men (98%) and women (78%). In most cohort studies, the association of smoking with gastric cancer was stronger in men than in women [3]. It has been observed in the past studies that proportionally more men are diagnosed with the intestinal histologic type of gastric cancer, while proportionally more women are diagnosed with the diffuse histologic type [13]. If there is a stronger association of cigarette smoking with the intestinal type, then this could account for some of the differences between men and women in the relation of smoking to gastric cancer. Our results with a limited number of cases suggest this possibility, as the hazard ratio was 2.06 for intestinal cancer and 1.18 for diffuse cancer. In two cohort studies in Japan among men, one reported a higher risk of smoking for the intestinal type [14] while the other study did not [15]. Clearly, more studies are needed to determine the association of cigarette smoking with the specific histological types of gastric cancer.
In a recent review of 42 cohort studies [3], the sex-specific summary-relative risks among current smokers were 1.62 in men (95% CI 1.50–1.75) and 1.20 in women (95% CI 1.01–1.43). Although this meta-analysis suggested that there may be a male–female difference in the magnitude of the association between smoking and gastric cancer, it was noted that the International Agency for Research against Cancer (IARC) concluded in its evaluation of carcinogenic risks to humans that the relative risks were similar for men and women in studies including adequate numbers of women [16].
In the same review [3], former male smokers had an increased risk of gastric cancer (RR = 1.34; 95% CI 1.22–1.47), but not former female smokers (RR = 1.16; 95% CI 0.92–1.46). Our results were similar. We also observed, as in other studies [3], that a shorter interval since quitting among former male smokers yielded a higher risk, even after taking cigarette-years into account. This provides further support for a positive relationship between cigarette smoking and gastric cancer. In addition, this initial report of a consistent positive association between current cigarette smoking and gastric cancer in five different ethnic groups strengthens the case for such an association.
Earlier studies investigating a dose–response relation between cigarette smoking and gastric cancer have produced equivocal results. In a meta-analysis in 1997 [4], a positive effect was reported in 6 of 30 case–control studies and in 4 of 10 cohort studies. A more recent meta-analysis in 2008 suggests that there is a dose–response trend, based on number of cigarettes per day [3]. The effect of duration of cigarette smoking was not assessed in the 2008 analysis. In the large European Investigation into Cancer and Nutrition (EPIC) study based on 10 European countries [5], hazard ratios for gastric cancer increased with intensity and duration of cigarette smoking. In our investigation, current male and female smokers who smoked more than 20 cigarettes a day had the highest gastric cancer risk. Also, there was a significant trend in gastric cancer risk with increasing years of cigarette smoking among both men and women, but the trend was not monotonic. Overall, our results support the presence of a weak dose–response trend between cigarette smoking and gastric cancer.
Although there were just 104 cases of cardia cancer in our study, we did find a positive association among ever cigarette smokers. When men and women were combined, both current and former cigarette smokers had a significantly higher risk for cardia cancer than they had for distal gastric cancer. In the EPIC Study, the relative risks among current smokers were 4.10 (95% CI 1.76–9.57) for cardia cancer and 1.94 (95% CI 1.05–3.60) for non-cardia gastric cancer [5]. Some additional studies have also suggested that cigarette smoking is a stronger risk factor for cardia than for distal gastric cancer [14, 17], but others have not supported this view [15, 18]. In a meta-analysis that included nine cohort studies, the summary-relative risks among current smokers were 1.87 (95% CI 1.31–2.67) for cardia cancer and 1.60 (95% CI 1.41–1.80) for non-cardia gastric cancer [3]. A factor that is contributing to the lack of consistency in showing that smoking may be more strongly associated with cardia than with distal gastric cancer is the extent of misclassification for cardia cancer [14]. Nonetheless, the case is strengthened that risk for both subsites is positively associated with cigarette smoking, supporting the impression that other factors are more important in accounting for the diverging trend in the risk for these sub-sites of gastric cancer.
One of the limitations in our study was that the lack of information on the H. pylori status of study participants. Because of this, we adjusted for place of birth which is potentially reflective of H. pylori prevalence. In a recent population-based cohort study of 1,071 Japanese men, it was found that there was a positive association of cigarette smoking with gastric cancer, even after adjusting for H. pylori infection. Furthermore, there was a marked increase in gastric cancer risk among subjects who had both a smoking habit and H. pylori infection compared with those who did not have both risk factors [19].
There are several mechanisms that could lead to an association between cigarette smoking and gastric cancer. Tobacco products contain a number of carcinogens that have been linked to gastric adenocarcinoma in humans [20]. Smoking-related DNA adducts that can bind to gastric mucosa DNA have been found in gastric cancers of smokers [21]. Cigarette smoking has been associated with an increase in risk for dysplasia and intestinal metaplasia that are precursor lesions of gastric cancer [22]. N-nitroso-compounds are present in cigarette smoke and may be involved in gastric carcinogenesis [23].
In conclusion, our data suggest the following: (1) the association of current cigarette smoking with gastric cancer is present in both men and women; (2) the effect is consistent across five different ethnic groups; (3) there is some support for a dose–response effect of smoking among both sexes; (4) the smoking effect is present for both cardia and distal gastric cancer, but it is stronger for cardia cancer; and (5) the association of current smoking with gastric cancer is stronger for the intestinal than the diffuse histologic type.
Acknowledgments
We wish to thank the participants of the MEC Study. The MEC Study is supported by grant R37 CA 54281 from the National Cancer Institute. The tumor registries are supported by the National Institutes of Health, Department of Health and Human Services (contracts N01-PC-35137 and N01-PC-35139).
Footnotes
Conflict of interest The authors have no financial relationship with the organization that sponsored the research. The authors declare they have no conflict of interest.
Contributor Information
Abraham M. Y. Nomura, Email: abe@cc.hawaii.edu, Epidemiology Program, University of Hawaii Cancer Center, University of Hawaii, Honolulu, HI 96813, USA.
Lynne R. Wilkens, Epidemiology Program, University of Hawaii Cancer Center, University of Hawaii, Honolulu, HI 96813, USA
Brian E. Henderson, Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA 90033, USA
Meira Epplein, Division of Epidemiology, Department of Medicine, Vanderbilt University, Nashville, TN 37203, USA.
Laurence N. Kolonel, Epidemiology Program, University of Hawaii Cancer Center, University of Hawaii, Honolulu, HI 96813, USA
References
- 1.Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533–543. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Ladeiras-Lopes R, Pereira AK, Nogueira A, Pinheiro-Torres T, Pinto I, Santos-Pereira R, Lunet N. Smoking and gastric cancer: systematic review and meta-analysis of cohort studies. Cancer Causes Control. 2008;7:689–701. doi: 10.1007/s10552-008-9132-y. [DOI] [PubMed] [Google Scholar]
- 4.Tredaniel J, Boffetta P, Buiatti E, Saracci R, Hirsch A. Tobacco smoking and gastric cancer: review and meta-analysis. Int J Cancer. 1997;72:565–573. doi: 10.1002/(sici)1097-0215(19970807)72:4<565::aid-ijc3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez CA, Pera G, AGuda A, Palli D, Krogh V, Veneis P, Tumino R, Panico S, Berglund G, Siman H, Nyren O, Agren A, et al. Smoking and the risk of gastric cancer in the European prospective investigation into cancer and nutrition (EPIC) Int J Cancer. 2003;107:629–634. doi: 10.1002/ijc.11426. [DOI] [PubMed] [Google Scholar]
- 6.Brown LM, Devesa SS. Epidemiologic trends in esophageal and gastric cancer in the United States. Surg Oncol Clin N Am. 2002;11:235–256. doi: 10.1016/s1055-3207(02)00002-9. [DOI] [PubMed] [Google Scholar]
- 7.Kolonel LN, Henderson BE, Hankin JH, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol. 2000;151:346–357. doi: 10.1093/oxfordjournals.aje.a010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zippin C, Lum D, Hankey BF. Completeness of hospital case reporting from the SEER program of the national cancer institute. Cancer. 1995;76:2343–2350. doi: 10.1002/1097-0142(19951201)76:11<2343::aid-cncr2820761124>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 9.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 10.Haiman CA, Stram DO, Wilkens LR, Pike MC, Kolonel LN, Henderseon BE, Le Marchand L. Ethnic and racial differences in the smoking-related risk of lung cancer. New Engl J Med. 2006;354:333–342. doi: 10.1056/NEJMoa033250. [DOI] [PubMed] [Google Scholar]
- 11.Therneau TM, Grambsch PM. Modeling survival data: extending the cox model. New York: Springer; 2001. [Google Scholar]
- 12.Leffrondre K, Abrahamowicz M, Siemiatycki J, Rachet B. Modeling smoking history: a comparison of different approaches. Am J Epidemiol. 2002;156:813–823. doi: 10.1093/aje/kwf122. [DOI] [PubMed] [Google Scholar]
- 13.Sipponen P, Correa P. Delayed rise in incidence of gastric cancer in females results in unique sex ratio (m/f): etiologic hypothesis. Gastric Cancer. 2002;5:213–219. doi: 10.1007/s101200200037. [DOI] [PubMed] [Google Scholar]
- 14.Sasazuki S, Sasaki S, Tsugane S. Cigarette smoking, alcohol consumption and subsequent gastric cancer risk by sub-site and histologic type. Int J Cancer. 2002;101:560–566. doi: 10.1002/ijc.10649. [DOI] [PubMed] [Google Scholar]
- 15.Koizumi Y, Tsubono Y, Nakaya N, Kuriyama S, Shibuya D, Matsuoka H, Tsuji I. Cigarette smoking and the risk of gastric cancer: a pooled analysis of two prospective studies in Japan. Int J Cancer. 2004;112:1049–1055. doi: 10.1002/ijc.20518. [DOI] [PubMed] [Google Scholar]
- 16.International Agency for Research on Cancer. Tobacco smoking and involuntary smoking. In: Sasco AJ, Secretan MB, editors. IARC monographs on the evaluation of carcinogenic risks to humans. Vol. 83. Lyon: IARC; 2004. [Google Scholar]
- 17.Freedman ND, Abnet CC, Leitzmann MF, Mouw T, Subar AF, Hollenbeck AR, Schatzkin A. A prospective study of tobacco, alcohol, and the risk of esophageal and gastric cancer subtypes. Am J Epidemiol. 2007;165:1424–1433. doi: 10.1093/aje/kwm051. [DOI] [PubMed] [Google Scholar]
- 18.Tran GD, Sun X-D, Abnet CC, Fan J-H, Dawsey SM, Dong Z-W, Mark SD, Qiao Y-L, Taylor PR. Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China. Int J Cancer. 2005;113:456–463. doi: 10.1002/ijc.20616. [DOI] [PubMed] [Google Scholar]
- 19.Shikata K, Doi Y, Yonemoto K, Arima H, Ninomiya T, Jubo M, Tanizaki Y, Matsumoto T, Iida M, Kiyohara Y. Population-based prospective study of the combined influence of cigarette smoking and Helicobacter pylori infection on gastric cancer incidence: the Hisayama study. Am J Epidemiol. 2008;168:1409–1415. doi: 10.1093/aje/kwn276. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman D, Hoffman I. Chemistry and toxicology. In: Burns D, Cummings , Hoffman D, editors. Cigars: health effects and trends, smoking and tobacco control monograph no. 9. National Cancer Institute, Bethesda, NIH publicaton no; 1998. pp. 98–4302. [Google Scholar]
- 21.Dyke GW, Craven JL, Hall R, Garner RC. Smoking-related DNA adducts in human gastric cancers. Int J Cancer. 1992;52:847–850. doi: 10.1002/ijc.2910520602. [DOI] [PubMed] [Google Scholar]
- 22.Kneller RW, You WC, Chang YS, Liu WD, Zhang L, Zhao L, Xu GW, Fraumeni JF, Jr, Blot WJ. Cigarette smoking and other risk factors for progression of precancerous stomach lesions. J Natl Cancer Inst. 1992;84:1261–1266. doi: 10.1093/jnci/84.16.1261. [DOI] [PubMed] [Google Scholar]
- 23.Mirvish SS. Role of N-nitroso compounds (NOC) and N-nitrosation in etiology of gastric, esophageal, nasopharyngeal and bladder cancer and contribution to cancer of known exposures to NOC. Cancer Lett. 1995;93:17–48. doi: 10.1016/0304-3835(95)03786-V. [DOI] [PubMed] [Google Scholar]