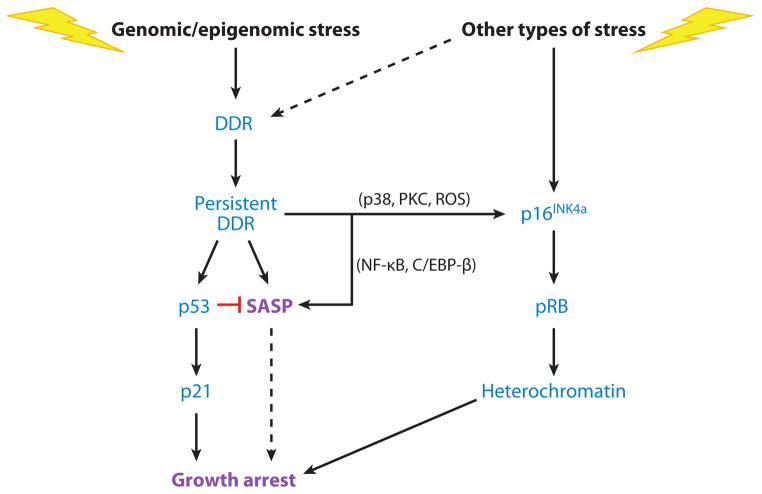
Figure 2.
Regulation of senescence growth arrest and the senescence-associated secretory phenotype (SASP). Cellular senescence is initiated by genomic or epigenomic damage, which activates a DNA damage response (DDR). The DDR ultimately becomes persistent or chronic, which leads to activation of p38MAPK and protein kinase C (PKC) and increased reactive oxygen species (ROS) and, ultimately, expression of the p16INK4a tumor suppressor. Stress that does not entail direct genomic or epigenomic damage can also induce p16INK4a expression and in some cases can indirectly trigger a DDR (dashed line). p16INK4a activates the pRB tumor suppressor, which silences certain proproliferative genes by heterochromatinization, thereby instituting a stringent arrest of cell proliferation. Persistent DDR signaling also induces the SASP and activates the p53 tumor suppressor, which restrains the SASP. p53 also causes growth arrest, principally by inducing expression of the cell cycle inhibitor p21. In some forms of oncogene-induced senescence, the SASP reinforces the senescence growth arrest (dashed line). NF-κB denotes nuclear factor κB.