Abstract
Objective
The current study assessed relations among maternal depressive symptoms, poorer youth diabetes adherence, and glycemic control. Specifically, hypothesized mediating links of lowered expectations of parental involvement, less parental monitoring and more conflict were examined.
Methods
Participants included 225 mothers and their young adolescents, aged 11–14 years (M = 12.73 years, SD = 1.2) diagnosed with T1D. Maternal depressive symptoms and outcome expectancies for maternal involvement were evaluated with self-report questionnaires. Multi-source, parent/youth, and multi-method assessment of adherence, parental monitoring, and conflict were evaluated during a baseline assessment from a larger randomized clinical trial.
Results
The first hypothesized structural equation model demonstrated a good fit and indicated that more maternal depressive symptoms were directly associated with less parental monitoring and more conflict, which in turn each were associated with poorer adherence and glycemic control. Although higher involvement expectancies were associated with more monitoring and less conflict, they were not associated with other model variables. A second alternative model also fit the data well; poorer youth adherence was associated with more conflict that in turn related to maternal depressive symptoms.
Conclusions
Two models were tested by which maternal depressive symptoms and poorer youth adherence were interrelated via less monitoring and more conflict. Follow-up longitudinal evaluation can best characterize the full extent of these relations.
Keywords: diabetes, maternal depression, adolescents, disease care, adherence
Type 1 diabetes (T1D) is a chronic illness that affects 1 in 500 youth in United States (Centers for Disease Control and Prevention, 2011). The rigorous regimen places high demands on parents and youths and relates to difficulties with psychosocial adjustment (Helgeson, Siminerio, Escobar & Becker, 2009). For mothers, disease-specific demands relate to elevated symptoms of depression (Driscoll et al., 2010; Eckshtain, Ellis, Kolmodin, & Naar-King, 2010) that equal or exceed national prevalence rates (e.g., Driscoll et al., 2010; Eckshtain et al., 2010), currently estimated around 10% (Erten, Rich-Edwards, & Koenen, 2011).
Maternal depression is directly linked to poorer youth psychosocial adjustment and disease care adherence (e.g., Jaser & Grey, 2010) as well as indirectly related to poorer youth glycemic control (Anderson et al., 2002; Eckshtain et al., 2010). Mechanisms of these relations have been partially explained. In young adolescents, maternal depression is associated with higher direct involvement in T1D management that is less beneficial than involvement from mothers without depressive symptoms (Wiebe et al., 2011). Parental depression has also been linked with poorer glycemic control via its association with general (not diabetes-specific) parental monitoring (Eckshtain et al., 2010). Moreover, parent-perceived disease burden mediates the relation between parent depression and poorer youth glycemic control (Cunningham, Vesco, Dolan, & Hood, 2011). However, despite the association of maternal depression with direct involvement, general monitoring, and parent-perceived disease burden, these factors do not fully explain the association with glycemic control. Other factors such as conflict, parental monitoring and expectancies regarding outcomes of parental monitoring may play an explanatory role in a more comprehensive understanding of optimal youth diabetes care.
Family conflict (general and diabetes-specific) is associated with poorer youth adherence and glycemic control (Anderson, et al., 2002; Wysocki et al., 2006). In the general population, maternal depression and family conflict have a transactional relation such that each worsens the other (Horwitz, Briggs-Gowan, Storfer-Isser, & Carter, 2007). Diabetes-specific family conflict also relates to maternal distress and poorer glycemic control (Williams, Laffel, & Hood, 2009).
The role of maternal involvement also merits consideration in youth diabetes care. Although previous research finds parental involvement remains high for mothers with depressive symptoms (Wiebe et al., 2011), the relation between depressive symptoms and involvement may depend on the type of parental involvement in disease care. For example, direct parental involvement that includes maternal responsibility for disease management tasks may remain high in mothers with depressive symptoms because mothers may lack the initiative or ability to transfer care to their adolescents (Wiebe et al., 2011). In contrast to direct parental involvement, indirect involvement entails parental monitoring that is less “hands-on” and consists of supervision of diabetes care tasks. More parental monitoring for diabetes care is associated with better adherence and glycemic control (e.g., Ellis, et al., 2007) and general (non-diabetes-specific) monitoring is associated with parental depression and glycemic control in youth with both T1D and T2D (Eckshtain et al., 2010). However, diabetes-specific monitoring has been relatively unstudied in relation to maternal depressive symptoms. Perhaps parental monitoring of adolescents’ diabetes self-care is more difficult for mothers with depressive symptoms and instead these mothers may simply opt to perform diabetes-related tasks for their youth. Although parental monitoring and family conflict are associated with one another and related to poorer glycemic control (Hilliard, Holmes, Chen, Maher, Robinson, & Streisand, 2012), the association of maternal depressive symptoms within this relation is yet to be examined, despite the relatively high prevalence of depressive symptomology in mothers of youth with T1D.
Finally, Social Cognitive Theory posits that one’s positive expectations about outcomes from one’s actions guide behavior (Bandura, 1997). For example, youths’ positive expectations about diabetes care are associated with better self-efficacy, adherence, and glycemic control (Iannotti et al., 2006). However, parental expectations of their involvement in care have not been examined despite the association between maladaptive cognitions and maternal depressive symptoms. The potential relevance is clear since parents who expect negative outcomes are less likely to be involved in their child’s care. Moreover, if family conflict is significant, mothers might be inclined to discount any beneficial effect of their participation in care.
To elucidate a comprehensive model of maternal depression and glycemic control in youth with T1D, the current study used structural equation modeling to evaluate two models. The first, symptom-initiated model, hypothesized that more maternal depressive symptoms would be associated with less monitoring of youth diabetes care and higher family conflict, both general and diabetes-specific, as well as poorer outcome expectations (hypothesized to be related to less monitoring and more conflict). Less monitoring and more conflict would in turn be related simultaneously to poorer adherence and glycemic control. Given the cross-sectional nature of the data, a second, adherence-initiated, model was evaluated which hypothesized poorer adherence would be associated with more depressive symptoms via more conflict and less monitoring, and poorer glycemic control. In this adherence-driven model, outcome expectations would mediate the association between more conflict and less monitoring within the model. The current study focused on young adolescents who are at-risk for poorer adherence and glycemic control based on their age, as this period presents good opportunity for prevention efforts.
Method
Participants and Procedures
Participants included 225 adolescents with T1D, ages 11 to 14 years (M = 12.73, SD = 1.2) and their mothers recruited from pediatric endocrinology clinics in two major metropolitan children’s hospitals. Participants who were accompanied by their mothers were from a larger sample of 257 adolescent- parent dyads who participated in a baseline evaluation as part of a larger randomized controlled trial (RCT). Participation in the RCT entailed completion of four brief sessions of behavioral intervention or diabetes information in conjunction with quarterly diabetes clinic visits over the course of 1–1½ years. The current study used data from the baseline assessment and included a second assay of glycemic control from the next sequential medical appointment, prior to initiation of intervention, approximately three months following baseline.
Eligibility criteria for the RCT included diabetes duration of at least one year, absence of severe complications or other medical diagnoses, and English fluency. Boys comprised 50.7% of the sample. The majority of families were Caucasian (69.6%; 18.5% African-American, 6.2% Hispanic, and 5.7% other). Most families were of upper-middle or middle-class socioeconomic status (SES) (Class I = 13.7%, Class II = 41.1%, Class III = 38.8%; Hollingshead, 1975). Married mothers to either a biological or step-parent comprised 77.3% of the participants, with the majority of those married to the youth’s biological parent. Within the current sample, 73.8% of adolescents used a flexible basal bolus regimen via insulin pump or injections. Average glycosylated hemoglobin (HbA1c) was 8.80% (SD = 1.61%), and mean illness duration was 5.11 years (SD = 3.01). Participant characteristics are summarized in Table 1.
Table 1.
Participant characteristics, N=225.
| % or M±SD | Adolescent | Parent | Range | |
|---|---|---|---|---|
| Youth Age (years) | 12.8 ±1.2 | 11–14 | ||
| Youth Gender, % female | 49.3 | |||
| Family Structure, % unmarried | 23.3 | |||
| Youth Ethnicity, % non-white | 30.4 | |||
| Hollingshead SES, % in 2 highest classes | 54.8 | |||
| Diabetes Duration (years) | 5.1 ± 3.0 | 1–13.6 | ||
| Maternal Depression (BDI-II) | 8.2 ± 7.7 | 0–44 | ||
| Parent Monitoring (PMDS) | 77.8 ± 7.9 | 78.4 ± 7.6 | 51.9–90 | |
| % of BG checks discussed/day (DI) | 61.6 ± 30.7 | 65.4 ± 28.5 | 0–100 | |
| Diabetes-Related Family Conflict (DFCS-R) | 27.5 ± 10.6 | 25.9 ± 7.0 | 19–57 | |
| General Family Conflict T-score (FES) | 55.1 ± 23.6 | 46.4 ± 11.8 | 33–80 | |
| Outcome Expectations (OEPI) | 47.9 ± 6.0 | 34–64 | ||
| Diabetes Management (DBRS) | 0.64 ± 0.13 | 0.67 ± 0.11 | .29–.98 | |
| Average frequency of BG checks/day (DI) | 3.7 ± 1.7 | 3.2 ± 1.9 | 0–8 | |
| HbA1c (%): at Assessment | 8.8±1.6 | 6.3–14 | ||
| 3-month post-assessment | 9.0±1.6 | 5.9–14 |
Following approval from the institutional review boards, eligible families were identified using clinic lists and mailed informational letters followed by telephone contact. 404 families were identified as eligible and 281 gave consent to participate (71%). Of the families who consented, 257 adolescent- parent dyads (91%) completed baseline assessments. 225 of those families (88%) had participating mothers who completed the assessment of depressive symptoms at baseline. Families who declined primarily cited lack of interest or time. Participants met with a research assistant at regularly scheduled diabetes clinic appointments and completed self-report questionnaires and a diabetes interview. A second interview was completed by phone within approximately two weeks. Families received a $25 gift card as remuneration.
Measures
Medical/demographic information
Mothers completed medical and demographic questionnaires to provide information regarding, ethnicity, parent marital status, and socioeconomic information. Additional medical information was obtained from medical charts. The Hollingshead Four Factor Index (Hollingshead, 1975) was used to calculate socioeconomic status (SES) category scores (categories 1–5), where lower scores indicate higher SES.
Glycemic control (HbA1c) was determined by blood assay at each site at assessment and three months post-assessment to provide greater measurement stability, using the same measurement technology at each site (DCA 2000), 4.3–5.7%, Bayer, Tarrytown, NY). Medical data were verified by a trained research assistant who reviewed the records.
Adherence
Parents and adolescents completed a 24-hour diabetes interview (DI; Holmes et al., 2006, adapted from Johnson, Silverstein, Rosenbloom, Carter, & Cunningham, 1986). The DI asks parents and adolescents separately to describe the completion of diabetes management behaviors, such as blood glucose (BG) checking and insulin administration. The DI was administered during an initial in-clinic assessment and again over the phone within 2 weeks. Each reporter’s responses were averaged across the two DI’s to create parent- and adolescent- reported variables. The 24-hour recall is a valid, reliable, and well-established measure of diabetes adherence behaviors (Quittner, Modi, Lemanek, Ievers-Landis, & Rapoff, 2008).
In the current study, DI responses to BG checking questions were used as a proxy measure of diabetes adherence that is linked with glycemic control (Hilliard et al., 2011). The present study assessed BG monitoring as total number of BG checks reported during a 24-hour period, averaged across the two days of recall for both parent and adolescent reports.
The Diabetes Behavior Rating Scale (DBRS; Iannotti, Nansel et al., 2006) was used as a comprehensive measure of adherence behaviors. The DBRS asks respondents to rate the frequency with which routine diabetes care behaviors occurred over the previous week. Participants completed either the multiple daily insulin injection form or the insulin pump form according to their prescribed regimen. Higher DBRS scores are indicative of more adherent behaviors. The DBRS has good internal consistency, content validity, and test-retest reliability (Iannotti, 2006); the internal consistency for the present sample was adequate (α= .79 for parents and adolescents on the injection form; parent α= .76 and adolescent α= .82 on the pump form).
Maternal depressive symptoms
The Beck Depression Inventory, Second Edition (BDI-II; Beck, Steer & Brown, 1996) was used to assess maternal depressive symptoms. In the current study, mothers who scored above a 29 were provided referral information for follow-up. The BDI-II is widely accepted as a screening measure of state depressive symptomology and has high internal consistency (α = .92–.93) and test-retest reliability (r = .93; Beck et al., 1996). Within the current sample, high internal consistency also was detected (α = .91).
Parental monitoring
The Parent Monitoring of Diabetes Scale (PMDS; Ellis et al., 2007) was used to measure parental monitoring of disease care behaviors. For the current analyses, the total scale was used (Ellis et al., 2008). Higher scores indicate higher levels of parental monitoring. The PMDS has good internal consistency (α = .81) and test-retest reliability (r = .80; Ellis et al., 2008), with adequate internal consistency in the current sample (α = .73).
From the DI, tallied across 2 typical disease care days, adolescents and their parents were asked whether a BG check and/or value was discussed with an adult for each and every check reported. This was considered an index of parental monitoring of disease care, as it was assumed that parents asking teens if they had checked their BG or for the value would represent parental monitoring. The percentage of BG checks discussed with an adult as reported both by a parent and by an adolescent separately was used as an index of daily parental monitoring of BG checks.
Family conflict
Family Environment Scale, Conflict subscale (FES; Moos & Moos, 2002). Parents and adolescents separately completed this self-report measure. Higher scores indicate higher perceived conflict. The measure has good reliability (Moos & Moos, 2002). In the current sample, internal consistency was adequate (parent α = .76, adolescent α = .74).
The Diabetes Family Conflict Sale- Revised (DFCS-R; Hood, Butler, Anderson, & Laffel, 2007) assessed parent-adolescent conflict regarding diabetes care behaviors. Mothers and adolescents described how much they argued about particular diabetes situations during the past month. The DFCS-R has good internal consistency, also seen in the current sample (parent α = .89; child α = .96). The total scores from the parent and adolescent report were used for analysis.
Outcome Expectations
The Outcome Expectations for Parental Involvement (OEPI; Iannotti, 2006) is a parent self-report measure of outcome expectations for parental involvement in their child’s diabetes management with adequate internal consistency (α = .84). The measure has good reliability and validity with adequate internal consistency in the current study (α = .80).
Data Analytic Plan
Using two models hypothesizing different pathways of influence, parental monitoring and family conflict were evaluated as mediators of the relations among maternal depressive symptoms, poorer youth adherence behaviors and poorer glycemic control. The contribution of outcome expectancies of parental involvement related to actual parental monitoring and its relation with family conflict also was evaluated. The models were analyzed using Structural Equation Modeling in Mplus 6 (Muthen & Muthen, 1998–2010). The full information maximum likelihood procedure was used to include participants who had individual non-demographic data points missing, presumed to be missing at random. This procedure estimates missing data values based on the current estimate of known parameters and then re-estimates the parameters based on known and imputed data (Collins et al., 2001). This is a preferred method for handling missing data, as it includes all available data in statistical analyses (Collins et al., 2001).
The data were screened for outliers and found to be within normal limits for skewness (≤3) and kurtosis (≤10; Kline, 2011). Model fit was assessed with a chi-square analysis, root-mean-square error of approximations (RMSEA; values below .06 indicate good fit; Kline, 2011), comparative fit index (CFI; values above .90 indicate acceptable fit; Hu & Bentler, 1998, 1999) and the standardized root mean square residual (SRMR; values less than .08 are acceptable; Kline, 2011). However, due to the large sample size of the current study, the chi-square value is not considered the best indicator of model fit (Kline, 2011). Instead, the CFI, RMSEA, and SRMR values are considered better indicators and were the focus of subsequent analyses.
Results
Descriptive Results
Approximately 21% of mothers (n = 47) reported clinically elevated symptoms of depression as indicated by a BDI-II score of 14 or greater, which is consistent with current research of mothers of adolescents with T1D (Driscoll et al., 2010; Horsch, McManus, Kennedy, & Edge, 2007) and mothers of younger children in the general pediatric population (22%; Dubowitz et al., 2007). Of the 21%, five mothers endorsed suicidal ideation and were referred for follow-up. Mothers who scored in the severe depressive range were also contacted and referred for follow-up. Of all mothers in the sample, 11.1% reported mild depressive symptoms, 7.6% reported moderate depressive symptoms and 2.2% reported severe depressive symptoms. For symptoms most frequently noted by severity, see Table 2.
Table 2.
Most frequently endorsed depressive symptoms on the BDI-II for all mothers N= 225
| BDI-II Severity Score |
||||
|---|---|---|---|---|
| Depressive Symptom | 0 | 1 | 2 | 3 |
| Less Energy | 29% | 58% | 13% | -- |
| Fatigue | 62% | 33% | 3% | 2% |
| Guilt | 62% | 33% | 4% | <1% |
| Loss of Pleasure | 62% | 33% | 3% | 2% |
Note. Severity Score relates to severity of endorsed depressive symptoms on a 4-point scale, higher scores indicate more severe symptoms. Percentages indicate the percentages of participants who endorsed depressive symptom at each level of severity.
Correlations among the variables of interest were examined (see Table 3 supplement online). Demographic variables that were significantly correlated with predictor, mediating, or outcome variables were included in the model regressed on the latent variables with which they were associated. As outcome expectations were not significantly associated with adherence or glycemic control, this pathway was eliminated from the evaluated model.
Models
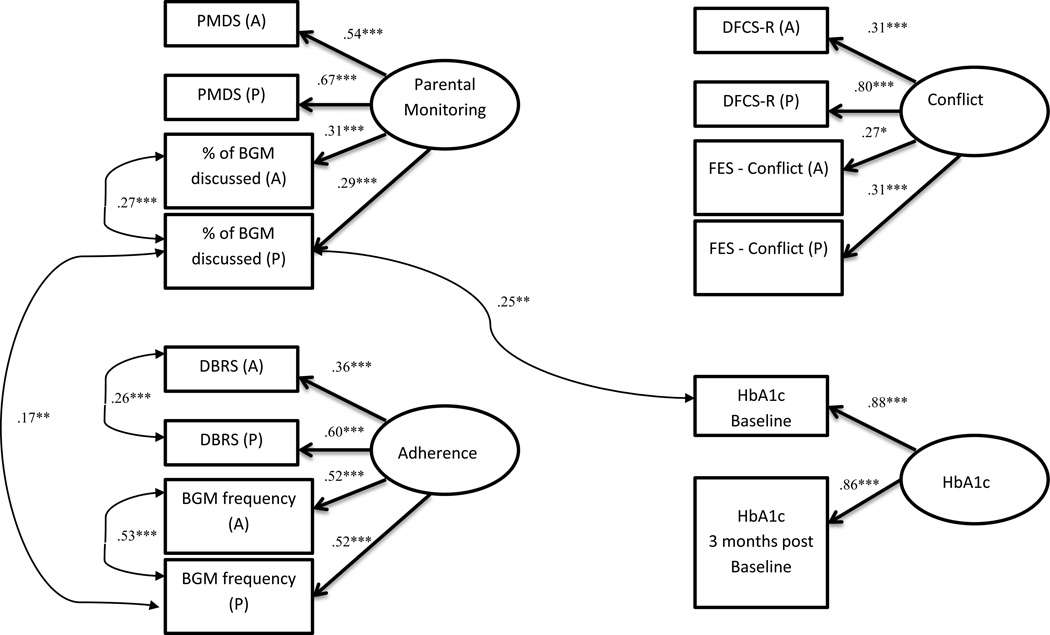
The hypothesized measurement model consists of four latent variables measuring parental monitoring, family conflict, adherence, and glycemic control. The proposed measurement model did not fit the data well, as indicated by all fit indices being outside of suggested ranges (χ2 (71) = 162.00, p<.001, CFI = .85, RMSEA = .08; 90% CI = .06–.09, SRMR = .09). However, all indicators sufficiently loaded onto the hypothesized latent variables (all factors: β>.26, p<.04). To improve model fit in accordance with MPlus suggested modification guidelines (Kline, 2011; MacCallum & Austin, 2000), correlations in error variances for parent and adolescent discussion of BG checks, parent and adolescent total BG checks, parent report of total BG checks and discussion of BG checks, A1c from baseline and parent discussion of BG checks, and parent and adolescent DMBS scores were added. These error variance correlations were theoretically sound based on shared method variance and the covariances were added to the model, which is a standard accepted practice in SEM (Kline, 2011). With these error variance correlations included, the measurement model evidenced a good fit for the data (χ2 (66) = 85.28, p=.06, CFI = .97, RMSEA = .04; 90% CI = .00–.06, SRMR = .07). See Figure 1, which includes all error variances. As with the first measurement model, all hypothesized indicators sufficiently loaded onto the hypothesized latent variables (all factors: β>.27, p<.03).
Figure 1.
Latent variable measurement models.
Note: *p<.05, **p<.01, ***p<.001 Add legend with acronyms
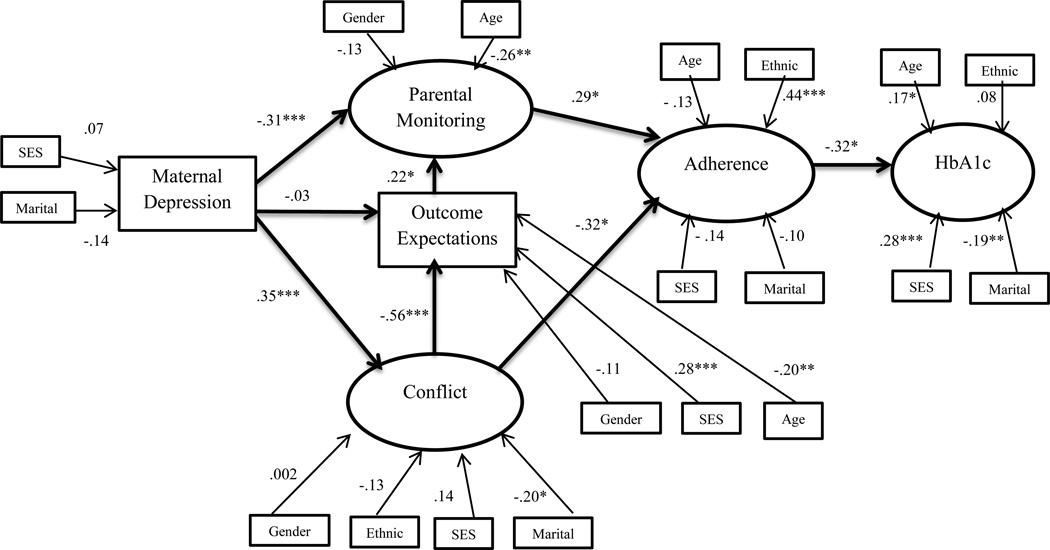
A hybrid model was evaluated that included covariates indicated by initial correlations with age, socioeconomic status, gender, ethnicity, and marital status. The resulting first model demonstrated good fit (χ2 (154) = 221.68, p<.001, CFI = .91, RMSEA = .05; 90% CI = .03–.06, SRMR = .07). See Figure 2. Maternal depressive symptoms were significantly associated with both more conflict (β = .35, p<.001) and less monitoring (β = −.31, p<.001) as hypothesized, but not with outcome expectations (β = −.03, p=.70). However, positive outcome expectations were associated with less conflict (β = −.56, p<.001) and more monitoring (β = .22, p=.02). In turn, more conflict (β = −.32, p=.02) and less monitoring (β = .29, p=.01) each was associated with poorer adherence as expected. Finally, poorer adherence was associated with poorer glycemic control (β = −.32, p=.02). The indirect path between depressive symptoms and adherence was significant (β = −.22, p<.001), but between maternal depressive symptoms and HbA1c demonstrated a trend towards significance (β = .07, p=.06). Of the demographic variables, older youth age was significantly associated with less parental monitoring (β = −.26, p=.002), less positive outcome expectations (β =-.20, p=.002), and poorer glycemic control (β = .17, p=.04). Single parenthood was associated with greater conflict (β = −.20, p=.03) and poorer glycemic control (β = −.19, p=.01). Adherence was better in white adolescents as compared to non-white adolescents (β = .44, p<.001). Higher socioeconomic categories were associated with better glycemic control (β = .28, p=.001) and more positive outcome expectations (β = .28, p<.001). This model accounted for 30% of the variance in family conflict, 29% of the variance in parental monitoring, 56% of the variance in adherence, and 34% of the variance in glycemic control.
Figure 2.
Standardized path coefficients in model 1 initiated with depressive symptoms
Note: Demographic and medical variables with significant bivariate correlations were included.
+ p <.10, * p<.05, ** p<.01, *** p<.001
Gender:0=male, 1=female; SES: lower values represent higher socioeconomic status; Ethnic: 0=non-white, 1=white; Marital: 0=not married, 1=married
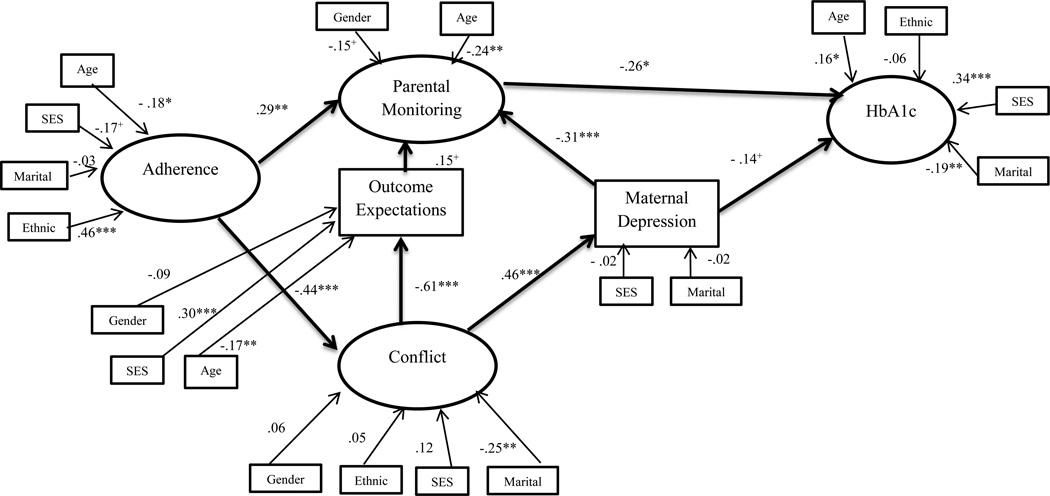
A second model was examined to evaluate the role of poorer youth adherence in relation to a range of adverse maternal, family and glycemic factors. This model also demonstrated a good fit of the data (χ2 (154) = 219.82, p<.001, CFI = .91, RMSEA = .05; 90% CI = .03–.06, SRMR = .07) and indicated poorer adherence is associated with higher conflict (β =−.44, p<.001) and less parental monitoring (β = .29, p<.01). Higher conflict was associated with more maternal depressive symptoms (β =.46, p<.001), which were in turn associated with less monitoring (β = −.31, p<.001). Within the model, less conflict again was associated with more positive outcome expectations (β = −.61, p<.001), which in turn demonstrated a trend towards being associated with more monitoring (β = .15, p=.09). Monitoring was associated with better glycemic control (β = −.26, p=.02), and maternal depressive symptoms demonstrated a trend towards association with better glycemic control (β = −.14, p=.10). None of the indirect pathways were significant. This second model accounted for 33% of the variance in conflict, 42% of the variance in parental monitoring, 31% of the variance in adherence, and 32% of the variance in glycemic control.
Discussion
The current study found 21% of mothers of adolescents with T1D had relatively high levels of depressive symptoms compared to a 10% prevalence rate of mothers from the general population (Erten et al., 2011). The current prevalence rate of 21% is consistent with a 10–33% prevalence rate among mothers of youth with T1D (Driscoll et al., 2010; Eckshtain et. al., 2009). In short, clinically elevated depressive symptoms are two to three times higher in mothers of youth with T1D. Among mothers who reported more severe depressive symptoms, the majority noted concerns with fatigue, guilt, and anhedonia. Of those with moderate symptoms, a third reported those same symptoms and low energy too.
Prior research shows a strong link among maternal depression, poorer adherence and poorer glycemic control in youth with T1D. The current study identifies parental monitoring as another key link among these variables. As predicted, higher maternal depressive symptoms were associated with less parental monitoring. Consistent parental monitoring in diabetes management is routinely identified as a necessary component of better glycemic control, particularly during adolescence (Helgeson et al., 2009). The current results extend this finding to a significant percentage of mothers who experience clinically elevated depressive symptoms.
Less parental monitoring in the present study may appear to represent a departure from previous research that shows maternal depressive symptoms associated with more direct parental involvement (Wiebe, 2011). However, other studies of parental involvement have focused on direct involvement such as task completion rather than indirect involvement via monitoring in the present study. Ironically, more direct parental involvement by mothers with depressive symptoms is actually less effective compared to that of mothers without symptoms (Wiebe et al., 2011), likely due to more negative and intrusive interactions (Jaser & Grey, 2010). A developmental transition to more youth autonomy occurs in early adolescence (Wysocki et al., 1992) as older adolescents assume more personal responsibility for diabetes-related tasks. A corresponding transition from direct to indirect parental involvement also occurs, characterized as monitoring or supervision (Schilling, Knafl, & Grey, 2006). Mothers who encourage and accomplish a smooth transfer of responsibility to older youth are arguably more responsive to different developmental expectations and needs. In contrast, mothers who experience depressive symptoms, specifically less energy and more fatigue, may find that these symptoms interfere with their ability to manage different needs of youth at different ages, especially during a period of rapid maturation. Thus, in the present study, more depressive symptoms were related to less parental monitoring and poorer adherence and glycemic control.
Adding to the understanding of the relation between maternal depressive symptoms and youth glycemic control, higher maternal depressive symptoms in the current study were related to more family conflict that was in turn associated with poorer adherence and poorer glycemic control. See Figure 2. The link between maternal depression and increased conflict may be explained in part by irritability, a frequent symptom of depression, which may make communication about diabetes and general family issues more conflicted. Other symptoms of depression, such as anhedonia or negative mood conceivably could create additional tensions within a family. Conflict, in turn, is associated with poorer adherence and glycemic control. Diabetes-specific conflict is consistently related to less frequent disease care behaviors (Anderson et al., 2002) and poorer glycemic control (Anderson et al., 2009), but for the first time is linked with maternal depressive symptoms in conjunction with general family conflict. Moreover, the second alternative model demonstrates that higher conflict is associated with more maternal depressive symptoms and extends findings of a transactional relation between conflict and depressive symptoms to families of youth with T1D.
Also for the first time, lower outcome expectations for parental involvement were associated with more conflict and less parental monitoring. This indicates an association between families with higher conflict and mothers who are less inclined to believe that their monitoring will be effective in diabetes management. In contrast, outcome expectancies for parental involvement were not related to maternal depressive symptoms, adherence, or glycemic control in mothers of youth with T1D in contrast to a link shown among adolescent expectations for diabetes management and adherence (Iannotti, Schneider et al., 2006). Adolescents assume greater diabetes management with older age and those who have positive expectations for outcomes could be anticipated to have better adherence in contrast to maternal expectations as parental involvement becomes more distal and related to monitoring not direct task performance. Therefore, although involvement expectancies are not an important component of the association between maternal depressive symptoms and glycemic control, maternal expectations were associated with other important aspects of care including parental monitoring and conflict. Longitudinal evaluation may provide a better means to determine a relation between expectations for parental involvement and depressive symptoms should one exist. Furthermore, expectancy-related results should be viewed within the framework that the content development and psychometric properties of this measure are as yet unpublished.
Inclusion of a second alternative conceptual model in the current study provides strong evidence that the relations among maternal depressive symptoms, parental monitoring, and family conflict are likely transactional. Diabetes management can be challenging for families and poorer adherence may be inescapably associated with greater diabetes- and family-related conflict. Conflict in turn might be construed as a personal failure by a mother who assumes a primary caretaking role, like those in the current sample who attended clinic appointments with their youth. Maternal depressive symptoms are in turn directly related to poorer youth glycemic control although it is important to note the indirect paths from adherence in this model were not significant. Maternal depressive symptoms also relate to less parental monitoring which relates to poorer glycemic control. Both models provided an equally good fit for the data, and together demonstrate multiple putative directions of associations between maternal depressive symptoms and youth adherence. Interestingly, the association of outcome expectations in both models remained consistent in its association with less family conflict and more parental monitoring. Future longitudinal research is needed to better evaluate the direction of these relations.
In this study, maternal depressive symptoms were related to more family and diabetes-related conflict along with less parental monitoring which together accounted for 56% of the variance in explaining youth adherence and 34% of the variance in explaining glycemic control. Clearly maternal depressive symptoms have multiple, and powerful, adverse associations with youth disease management such that youth health care should involve screening and referral for maternal symptoms and attention to which aspects of home diabetes care are associated with greatest disruption. Regardless of the direct or indirect effects of maternal depressive symptoms on youth disease care and family conflict, clinical exploration of multiple aspects of disease care are indicated given the unfavorable associations universally reported.
As expected, less parental monitoring occurs with older adolescents. White adolescents reported better adherence than non-white adolescents, consistent with the literature (Swift, Chen, Hershberger, & Holmes, 2006). Finally, consistent with the literature, better glycemic control was associated with higher socioeconomic status and having a mother who was married (e.g., Swift et al., 2006). Ethnicity, socioeconomic status, and marital status are intertwined and contribute to self-care and glycemic control via a number of pathways. Awareness of these patterns can better inform clinical care.
The current study has a number of strengths, including use of multi-method, multi-informant variables and use of advanced statistical techniques to allow examination of complex interrelations among variables. However, these data are cross-sectional with the exception of glycemic control, measured both at time of assessment and three months later such that causal relations among the variables cannot be determined. Further, maternal depressive symptoms were measured with a single self-report questionnaire and follow-up of severe depressive symptoms. Future research can benefit through more thorough measurement of maternal depression. Participants were part of a larger randomized controlled trial, and, as such, may not be representative of larger clinic populations. Finally, other mechanisms related to maternal depressive symptoms, for example, parenting style, communication, and child psychopathology, may be associated with disease care and glycemic control and merit consideration in future research.
The relations among maternal depressive symptoms, diabetes care and glycemic control in adolescents with T1D continues to highlight the benefit of routine screening of depressive symptoms among mothers. Pediatric diabetes providers have a unique opportunity to identify mothers and refer depressive symptoms for treatment. Even brief training in physician-parent communication skills may have a positive impact on parental mental health (Wissow et al., 2008). Future research will be needed to determine how routine screening and referral for treatment might translate into specialized clinical care environments and whether treatment of maternal depression indeed leads to better outcomes in youth with T1D, including glycemic control and health care utilization. Careful parent screening for personal or family history of depression at time of youth diagnosis may be another effective tool to identify those with preexisting risk factors for depressive symptoms.
Conclusion
The current study confirms a significant prevalence of elevated levels of maternal depressive symptoms which may affect as many as 1 of 5 mothers of adolescents with T1D. The current study adds to existing literature through demonstration of associations of elevated depressive symptoms with lower parental monitoring and increased family conflict, each of which are associated with poorer disease care behaviors and glycemic control. Future research would benefit from an in-depth study of depressive symptoms experienced by mothers of adolescents with T1D. Qualitative research to determine specific triggers and stressors for mothers would provide further information about how symptoms may impact parental monitoring and family conflict.
Supplementary Material
Figure 3.
Standardized path coefficients in model 2 initiated with adherence.
Note: Demographic and medical variables with significant bivariate correlations were included.
+ p <.10, * p<.05, ** p<.01, *** p<.001
Gender:0=male, 1=female; SES: lower values represent higher socioeconomic status; Ethnic: 0=non-white, 1=white; Marital: 0=not married, 1=married
Acknowledgments
This work was supported by NIH/NIDDK 5R01DK070917-04 awarded to CH. The authors gratefully acknowledge the assistance of Elizabeth Robinson with manuscript preparations.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed., Text Revision. Washington DC: American Psychiatric Publishing, Inc.; 2000. [Google Scholar]
- Anderson BJ, Vangsness L, Connell A, Butler D, Goebel-Fabbri A, Laffel LM. Family conflict, adherence, and glycemic control in youth with short duration type 1 diabetes. Diabetic Medicine: A Journal of the British Diabetic Association. 2002;19:635–642. doi: 10.1046/j.1464-5491.2002.00752.x. [DOI] [PubMed] [Google Scholar]
- Anderson BJ, Holmbeck G, Iannotti RJ, McKay SV, Lochrie A, Volkening LK, Laffel L. Dyadic measures of the parent-child relationship during the transition to adolescence and glycemic control in children with type 1 diabetes. Families, Systems & Health: The Journal of Collaborative Family Healthcare. 2009;27:141–152. doi: 10.1037/a0015759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A. Self-efficacy: The exercise of Control. New York: W.H. Freeman; 1997. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Centers for Disease Control and Prevention. National diabetes fact sheet: National estimates and general information on diabetes and prediabetes in the United States, 2011. 2011 Retrieved from http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf.
- Collins LM, Schafer JL, Kam C. A comparison of inclusive and restrictive strategies in modern missing data procedures. Psychological Methods. 2001;6:330–351. [PubMed] [Google Scholar]
- Cunningham NR, Vesco AT, Dolan LM, Hood KK. From caregiver psychological distress to adolescent glycemic control: the mediating role of perceived burden around diabetes management. Journal of Pediatric Psychology. 2011;36:196–205. doi: 10.1093/jpepsy/jsq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll KA, Johnson SB, Barker D, Quittner AL, Deeb LC, Geller DE, Silverstein JH. Risk factors associated with depressive symptoms in caregivers of children with type 1 diabetes or cystic fibrosis. Journal of Pediatric Psychology. 2010;35:814–822. doi: 10.1093/jpepsy/jsp138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubowitz H, Feigelman S, Lane W, Prescott L, Blackman K, Grube L, Tracy JK. Screening for depression in an urban pediatric primary care clinic. Pediatrics. 2007;119:435–443. doi: 10.1542/peds.2006-2010. [DOI] [PubMed] [Google Scholar]
- Eckshtain D, Ellis DA, Kolmodin K, Naar-King S. The effects of parental depression and parenting practices on depressive symptoms and metabolic control in urban youth with insulin dependent diabetes. Journal of Pediatric Psychology. 2009;35:426–435. doi: 10.1093/jpepsy/jsp068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis DA, Podolski CL, Frey M, Naar-King S, Wang B, Moltz K. The role of parental monitoring in adolescent health outcomes: Impact on regimen adherence in youth with type 1 diabetes. Journal of Pediatric Psychology. 2007;32:907–917. doi: 10.1093/jpepsy/jsm009. [DOI] [PubMed] [Google Scholar]
- Ellis DA, Templin T, Podolski CL, Frey M, Naar-King S, Moltz K. The parental monitoring of diabetes care scale: Development, reliability and validity of a scale to evaluate parental supervision of adolescent illness management. Journal of Adolescent Health. 2008;42:146–153. doi: 10.1016/j.jadohealth.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Ertel KA, Rich-Edwards JW, Koenen KC. Maternal depression in the United States: Nationally representative rates and risks. Journal of Women’s Health. 2011;20:1609–1617. doi: 10.1089/jwh.2010.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgeson VS, Siminerio L, Escobar O, Becker D. Predictors of metabolic control among adolescents with diabetes: A 4-year longitudinal study. Journal of Pediatric Psychology. 2009;34:254–270. doi: 10.1093/jpepsy/jsn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard M, Holmes C, Chen R, Maher K, Robinson E, Streisand R. Disentangling the role of family conflict in adolescents’ management of type 1 diabetes. Health Psychology. 2013;32(4):388–396. doi: 10.1037/a0027811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. New Haven, CT: Yale University; 1973. Unpublished manuscript. [Google Scholar]
- Holmes CS, Chen R, Streisand R, Marschall DE, Souter S, Swift EE, Peterson CC. Predictors of youth diabetes care behaviors and metabolic control: A structural equation modeling approach. Journal of Pediatric Psychology. 2006;31:770–784. doi: 10.1093/jpepsy/jsj083. [DOI] [PubMed] [Google Scholar]
- Hood KK, Butler DA, Anderson BJ, Laffel LM. Updated and revised diabetes family conflict scale. Diabetes Care. 2007;30:1764–1769. doi: 10.2337/dc06-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsch A, McManus F, Kennedy P, Edge J. Anxiety, depressive, and posttraumatic stress symptoms in mothers of children with type 1 diabetes. Journal of Traumatic Stress. 2007;20:881–891. doi: 10.1002/jts.20247. [DOI] [PubMed] [Google Scholar]
- Horwitz SM, Briggs-Gowan MJ, Storfer-Isser A, Carter AS. Prevalence, correlates, and persistence of maternal depression. Journal of Women’s Health. 2007;16:678–691. doi: 10.1089/jwh.2006.0185. [DOI] [PubMed] [Google Scholar]
- Ianonotti R. Outcome Expectations for Parental Involvement. 2006 Personal communication. [Google Scholar]
- Iannotti RJ, Nansel TR, Schneider S, Haynie DL, Simons-Morton B, Sobel DO, Clark L. Assessing regimen adherence of adolescents with type 1 diabetes. Diabetes Care. 2006;29:2263–2267. doi: 10.2337/dc06-0685. [DOI] [PubMed] [Google Scholar]
- Iannotti RJ, Schneider S, Nansel TR, Haynie D, Plotnick L, Clark LM, Simons-Morton B. Self-efficacy, outcome expectations, and diabetes self-management in adolescents with type 1 diabetes. Journal of Developmental & Behavioral Pediatrics. 2006;27:98–105. doi: 10.1097/00004703-200604000-00003. [DOI] [PubMed] [Google Scholar]
- Jaser S, Grey M. A pilot study of observed parenting and adjustment in adolescents with type 1 diabetes and their mothers. Journal of Pediatric Psychology. 2010;35:738–747. doi: 10.1093/jpepsy/jsp098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SB, Silverstein J, Rosenbloom A, Carter R, Cunningham W. Assessing daily management in childhood diabetes. Health Psychology. 1986;5:545–564. doi: 10.1037/0278-6133.5.6.545. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. 3rd ed. New York: Guilford Press; 2011. [Google Scholar]
- MacCallum RC, Austin JT. Applications of structural equation modeling in psychological research. Annual Review of Psychology. 2000;51:201–226. doi: 10.1146/annurev.psych.51.1.201. [DOI] [PubMed] [Google Scholar]
- Muthen B, Muthen L. Mplus User's Guide. Sixth Edition. Los Angeles, CA: Muthen & Muthen; 1998–2010. [Google Scholar]
- Moos R, Moos B. Family Environment Scale Manual: Development, Applications, Research - Third Edition. Palo Alto, CA: Consulting Psychologist Press; 1994. [Google Scholar]
- Quittner AL, Modi AC, Lemanek KL, Ievers-Landis CE, Rapoff MA. Evidence-based assessment of adherence to medical treatments in pediatric psychology. Journal of Pediatric Psychology. 2008;33:916–936. doi: 10.1093/jpepsy/jsm064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling LS, Knafl KA, Grey M. Changing patterns of self-management in youth with type 1 diabetes. Pediatric Nursing. 2006;21:412–424. doi: 10.1016/j.pedn.2006.01.034. [DOI] [PubMed] [Google Scholar]
- Swift E, Chen RS, Hershberger A, Holmes CS. Demographic risk factors, mediators and moderator in youths diabetes metabolic control. Annals of Behavioral Medicine. 2006;32:355–365. doi: 10.1207/s15324796abm3201_5. [DOI] [PubMed] [Google Scholar]
- Wiebe DJ, Berg CA, Gelfand D, Butler J, Korbel C, Fortenberry KT, McCabe J. Longitudinal associations of maternal depressive symptoms, maternal involvement, and diabetes management across adolescence. Journal of Pediatric Psychology. 2011;36:837–846. doi: 10.1093/jpepsy/jsr002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LB, Laffel L, Hood KK. Diabetes-specific family conflict and psychological distress in pediatric Type 1 diabetes. Diabetic Medicine. 2009;26:908–914. doi: 10.1111/j.1464-5491.2009.02794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissow LS, Gadomski A, Roter D, Larson S, Brown J, Zachary C, Wang M. Improving child and parent mental health in primary care: A cluster-randomized trial of communication skills training. Pediatrics. 2008;121:266–275. doi: 10.1542/peds.2007-0418. [DOI] [PubMed] [Google Scholar]
- Wysocki T, Harris MA, Buckloh LM, Mertlich D, Lochrie AS, Taylor A, White NH. Effects of behavioral family systems therapy for diabetes on adolescents' family relationships, treatment adherence, and metabolic control. Journal of Pediatric Psychology. 2006;31:989–938. doi: 10.1093/jpepsy/jsj098. [DOI] [PubMed] [Google Scholar]
- Wysocki T, Meinhold PA, Abrams KC, Barnard MU, Clarke WL, Bellando BJ, Bourgeois MJ. Parental and professional estimate of self-care independence of children and adolescents with IDDM. Diabetes Care. 1992;15:48–52. doi: 10.2337/diacare.15.1.43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.