Abstract
Introduction
This study aimed at estimating the incidence of pregnancy after antiretroviral therapy (ART) initiation in eight West African countries over a 10-year period.
Methods
A retrospective analysis was conducted within the international database of the IeDEA West Africa Collaboration. All HIV-infected women aged <50 years and starting ART for their own health between 1998 and 2011 were eligible. Pregnancy after ART initiation was the main outcome and was based on clinical reporting. Poisson regression analysis accounting for country heterogeneity was computed to estimate first pregnancy incidence post-ART and to identify its associated factors. Pregnancy incidence rate ratios were adjusted on country, baseline CD4 count and clinical stage, haemoglobin, age, first ART regimen and calendar year.
Results
Overall 29,425 HIV-infected women aged 33 years in median [Inter Quartile Range: 28–38] contributed for 84,870 women-years of follow-up to this analysis. The crude incidence of first pregnancy (2,304 events) was 2.9 per 100 women-years [95% confidence interval [CI]: 2.7–3.0], the highest rate being reported among women aged 25–29 years: 4.7 per 100 women-years; 95% CI: 4.3–5.1. The overall Kaplan-Meier probability of pregnancy occurrence by the fourth year on ART was 10.9% (95% CI: 10.4–11.4) and as high as 28.4% (95% CI: 26.3–30.6) among women aged 20–29 years at ART initiation.
Conclusion
The rate of pregnancy occurrence after ART initiation among HIV-infected women living in the West Africa region was high. Family planning services tailored to procreation needs should be provided to all HIV-infected women initiating ART and health consequences carefully monitored in this part of the world.
Keywords: HIV, incident pregnancy, predicting factors, post-ART initiation, Africa
Introduction
Women of reproductive age constitute the largest population affected by HIV infection in sub-Saharan Africa (1). The wide availability of antiretroviral therapy (ART) in African settings has considerably decreased morbidity and mortality of many women living with HIV, reducing as well the risk of mother-to-child transmission of HIV (MTCT) (1, 2). We hypothesize that this outstanding improvement of life expectancy of women of childbearing age is positively associated with the increasing procreation desires and fertility rates observed after ART initiation in several settings (3–5). However, safe motherhood needs are partially met for HIV-infected women, since for instance the access to family planning, antenatal and postnatal care services of good quality are not fully available in most settings (6–11). Several studies have showed that fertility desire among childless HIV-affected couples, even if lower than in the general population, is high, especially among younger individuals (12, 13). Indeed, the pregnancy rate among HIV-infected women increases significantly after ART initiation, and this increase seems proportional to the duration of ART exposure, independently of the use of contraceptive methods (12–15).
The pregnancy occurrence among HIV-infected women remains a major clinical and public health concern for the following reasons. First, women starting ART for their own health during pregnancy present a higher risk of virological failure than women starting ART while not pregnant (16). Moreover, HIV-infection has been pointed out as a leading indirect cause of maternal death in sub-Saharan Africa (17–20), increasing the risk of several maternal complications positively associated with maternal death. (21, 22). Finally, the vertical risk of HIV transmission has not been eliminated yet, especially in lower income settings. This is primarily due to the failure to reach high levels of linkage and retention throughout the cascade of HIV services for all pregnant, delivering and breastfeeding women (23).
In West Africa, although the roll out of ART over the last decade has been slower than elsewhere in the developing world, it has considerably contributed to the overall improvement of survival of HIV-infected women in a context of high fertility. Available information about reproductive health patterns and more specifically demographic trends of fertility among HIV-infected women on ART remains poor in this region however. In this context, the aim of the present study was to estimate over a ten-year period the incidence of pregnancy after ART initiation on a large sample of ART-treated HIV-infected women of reproductive age, and to identify the associated factors of pregnancy occurrence after ART initiation in West-Africa.
Methods
The IeDEA West Africa collaboration
The International epidemiological Database to Evaluate AIDS (IeDEA) initiative (http://www.iedea-hiv.org) is a consortium of leading clinicians and epidemiologists launched in 2006 that has been addressing high priority research questions and streamline HIV/AIDS research through large pooled regional databases. Its African organization was extensively described elsewhere (24). This cohort analysis was conducted within the IeDEA West Africa Collaboration (http://mereva.isped.u-bordeaux2.fr/iedea/Accueil.aspx). Fifteen HIV/AIDS adult clinics located within eight countries participated in this report: Benin (n=1), Burkina Faso (n=2), Côte d'Ivoire (n=5), Gambia (n=1), Guinea Bissau (n=1), Mali (n=2), Nigeria (n=2) and Senegal (n=1). Every 18 months, each cohort submits information to the central coordinating center based in Côte d'Ivoire, using a standardized data format. The data collected capture demographic, clinical, biological and therapeutic information at baseline and during follow-up visits.
Inclusion/exclusion criteria and study sample
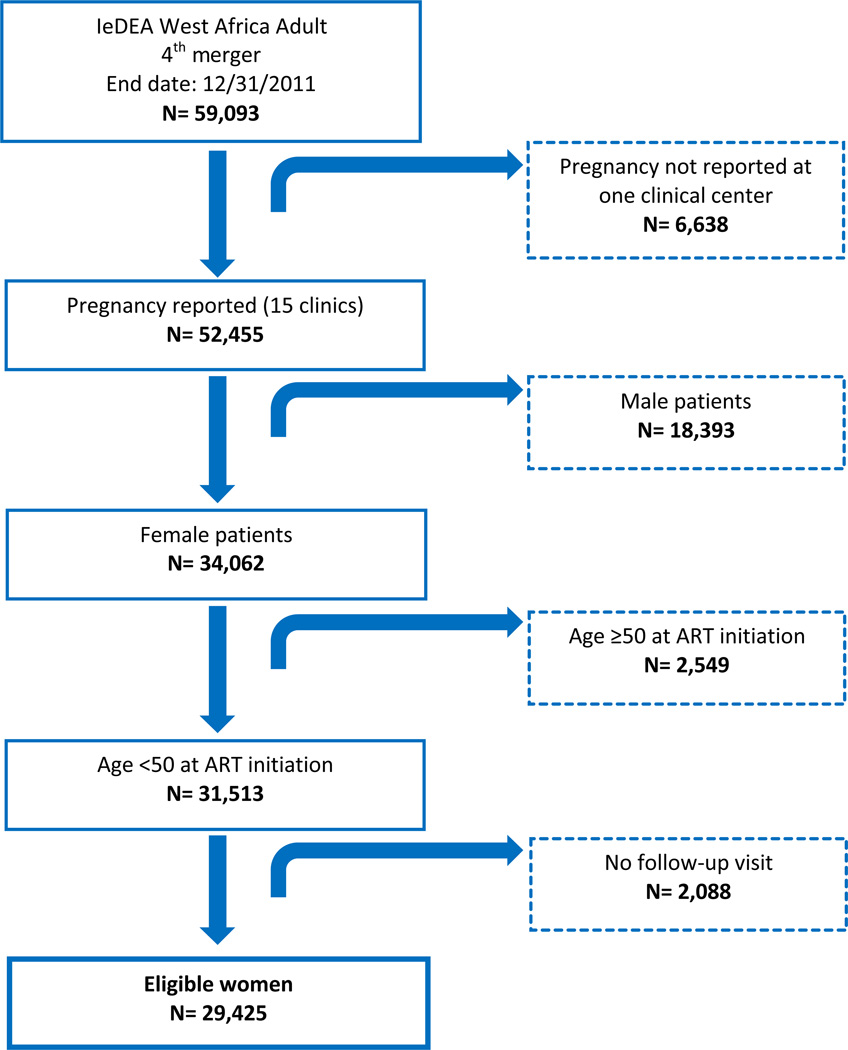
We conducted a retrospective analysis within the West-African cohort data base among all HIV-infected women of reproductive age (<50 years old) and starting ART for their own health between January 1998 and December 2011 according to in-country ART protocols. Women not reporting any follow-up visit were not retained for this analysis. Additionally, one clinic participating to the consortium was not systematically reporting pregnancies within its database and was therefore excluded from this analysis. As we were interested in estimating the incidence of pregnancy after ART initiation, HIV-infected women starting ART for their own health while pregnant were included for this analysis but were left-censored nine months after ART initiation (any prevalent pregnancy that initiated prior to ART initiation was thus not taken into account). The flow diagram of patients’ selection is detailed in Figure 1.
Figure 1.
Flow-chart of women eligible for the pregnancy analysis. The IeDEA West Africa Collaboration database, 1998–2011.
Data collection and Outcome definition
All eligible women started ART for their own health fulfilling clinical eligibility criteria established by in-country ART protocols. All of the participating clinics had the capacity to perform CD4 cell counts, hematology and biochemistry. ART was provided free of charge by the national treatment programs according to their individual treatment algorithms. After entry into care, women were typically followed every six months, or were seen at in-between visits for any inter-current illness. CD4-cell counts were measured every six months. Baseline CD4 was the measure performed at the time of ART initiation or in the previous six months. Women not seen at least six months without any transfer or death report were considered lost to follow-up.
Pregnancy diagnosis was considered based on the delay of the last menstruation period according to the woman self-report that was assessed at each follow-up visit. The main outcome measure for this analysis was ever becoming pregnant after ART initiation, as reported in the database.
Statistical analysis
Baseline characteristics were described and compared between pregnant and non-pregnant women using Chi-square test for categorical and qualitative variables and Kruskal-Wallis non-parametric test for continuous variables. Incidence rates of pregnancy event including recurrences were calculated per 100 women-years of follow-up with their 95% Confidence Interval (95% CI). Poisson regression method was used to estimate incident rate of first pregnancy among women of reproductive age starting ART as soon as January 15th, 1998 until endpoint date set at December 31st, 2011. Each woman included contributed to the denominator from time of ART initiation to their date of pregnancy recording, their last visit or December 31st 2011. Women were right-censored at the time of death or loss to follow-up when these events occurred.
Considering pregnancy as a time-dependent event, the probability of becoming pregnant according to the age at ART initiation was estimated using Kaplan-Meier analysis. To identify associated factors of the first pregnancy occurrence after ART initiation, we ran univariable and multivariable Poisson regression models performed with a manual backward selection method, assuming significant all associations with p-values <0.05. Maternal age was computed as a time dependent variable in Poisson regression models. ART history (pre-treated or naïve) was considered as an explanatory variable. Statistical analyses were generated using SAS software (version 9.2 for Windows, Copyright 2013 for SAS Institute Inc., Cary, NC, USA).
Results
Overall 29,425 HIV-infected women accounted for the present analysis, contributing a total of 84,870 women-years of follow-up with 4.4% of death and 25.6% of loss to follow-up across the study period.
Baseline characteristics according to pregnancy experience are detailed in Table 1. Women who reported a pregnancy were significantly younger at ART initiation than those who never reported such an event: 29.7 years in median vs. 33.5 years (p<0.001). Similarly, women who were identified as pregnant, started ART at higher median CD4 cell count level (191 cells/mm3) than those who never reported a pregnancy during the study period (172 cells/mm3) (p<0.001). Pregnancy was less frequent among women who started ART with a clinical stage III/IV or AIDS compared to those starting ART with a less advanced stage of HIV disease (19.6% vs. 24.5%) (p<0.001). Hemoglobin level at baseline among women becoming pregnant after ART initiation was slightly higher in median than among those who never became pregnant (10.2 g/dl vs. 10.1 g/dl; p=0.01).
Table 1.
Baseline characteristics according to women’s experience of pregnancy. The IeDEA West Africa Collaboration, 1998–2011.
| Characteristics at ART initiation |
Women ever becoming pregnant (N=2,304) |
Women never becoming pregnant (N=27,121) |
Total (N=29,425) |
p-value | |
|---|---|---|---|---|---|
| Age in years, median (IQR†) | 29.7 (26.5–32.9) | 33.5 (28.9–39.3) | 33.1 (28.6–38.8) | <0.0001* | |
| Baseline CD4 count (cells/µl), median (IQR) | 191 (108–285) | 172 (84–271) | 173 (86–272) | <0.0001* | |
| Baseline CD4 count (cells/µl) (%) | <0.0001** | ||||
| <50 | 194 (8.4) | 3,205 (11.8) | 3,399 (11.6) | ||
| 50–99 | 193 (8.4) | 2,645 (9.8) | 2,838 (9.6) | ||
| 100–199 | 527 (22.9) | 5,763 (21.3) | 6,290 (21.4) | ||
| 200–299 | 430 (18.7) | 4,399 (16.2) | 4,829 (16.4) | ||
| 300–349 | 117 (5.1) | 1,329 (4.9) | 1,446 (4.9) | ||
| 350–499 | 143 (6.2) | 1,453 (5.4) | 1,596 (5.4) | ||
| ≥500 | 119 (5.2) | 1,201 (4.4) | 1,320 (4.5) | ||
| Missing | 581 (25.2) | 7,126 (26.3) | 7,707 (26.2) | ||
| Hemoglobin (g/dl), median (IQR) | 10.2 (9.0–11.4) | 10.1 (8.9–11.3) | 10.2 (8.9–11.3) | 0.0130* | |
| Baseline hemoglobin (g/dl) (%) | 0.0028** | ||||
| ≥12 | 217 (9.4) | 2,417 (8.9) | 2,634 (9.0) | ||
| [10–12[ | 629 (27.3) | 6,288 (23.2) | 6,917 (23.5) | ||
| [8–10[ | 455 (19.8) | 5,155 (19.0) | 5,610 (19.1) | ||
| <8 | 147 (6.4) | 2,070 (7.6) | 2,217 (7.5) | ||
| Missing | 856 (37.2) | 11,191 (41.3) | 12,047 (40.9) | ||
| WHO or CDC clinical stage (%) | <0.0001** | ||||
| A,B/I,II | 1,574 (68.3) | 16,617 (61.3) | 18,191 (61.8) | ||
| AIDS/III,IV | 452 (19.6) | 6,648 (24.5) | 7,100 (24.1) | ||
| Missing | 278 (12.1) | 3,856 (14.2) | 4,134 (14.0) | ||
| Regimen at ART initiation (%) | <0.0001** | ||||
| 2NRTIs¥+Nevirapine | 1,665 (72.3) | 16,584 (61.1) | 18,249 (62.0) | ||
| 2NRTIs+Efavirenz | 416 (18.1) | 7,692 (28.4) | 8,108 (27.6) | ||
| 2NRTIs+PI§ | 156 (6.8) | 2,115 (7.8) | 2,271 (7.7) | ||
| 3NRTIs | 25 (1.1) | 359 (1.3) | 384 (1.3) | ||
| Other regimens | 42 (1.8) | 371 (1.4) | 413 (1.4) | ||
| History of ART (%) | 0.3622** | ||||
| Naïve | 2,142 (93.0) | 25,347 (93.5) | 27,489 (93.4) | ||
| Pre-treated | 162 (7.0) | 1,774 (6.5) | 1,936 (6.6) | ||
Interquartile range
Nucleoside reverse transcriptase inhibitors
Protease Inhibitors
Kruskal-Wallis test
Chi square test
ART: antiretroviral therapy
CDC: US Centers for Disease Control
WHO: World Health Organization
Pregnancy occurrence
As detailed in Table 2, 2,515 pregnancies were reported among 2,304 women during the course of follow-up: 2,107 women had one pregnancy, 183 had two pregnancies and 14 women reported three pregnancies. The crude incidence rate of pregnancy was 2.96 pregnancies per 100 women-years (95% CI: 2.85–3.08) (Table 2). The highest rate was reported in Burkina-Faso (3.46 pregnancies per 100 women-years; 95% CI: 3.11–3.81), followed by Nigeria (3.43 pregnancies per 100 women-years; 95% CI: 3.19–3.66) and Togo (3.35 pregnancies per 100 women-years; 95% CI: 2.57–4.13). For the remaining five countries, the incidence rate of pregnancy was below 3.0 pregnancies per 100 women-years.
Table 2.
Pregnancy incidence according to country and women’s baseline characteristics. The IeDEA West Africa Collaboration, 1998–2011.
| Country | Number of women (%) |
Number of first pregnancies |
Incidence of first pregnancy per 100 women- years (95% CI) |
Overall number of pregnancies |
Pregnancy incidence per 100 women- years (95% CI) |
|
|---|---|---|---|---|---|---|
| Country | ||||||
| Côte d’Ivoire | 10,107 (34.4) | 777 | 2.52 (2.34–2.70) | 887 | 2.71 (2.53–2.89) | |
| Benin | 1,621 (5.5) | 101 | 2.21 (1.78–2.64) | 119 | 2.41 (1.98–2.84) | |
| Burkina Faso | 3,685 (12.5) | 341 | 3.33 (2.97–3.68) | 379 | 3.46 (3.11–3.81) | |
| Guinea Bissau | 1,130 (3.8) | 20 | 1.20 (0.68–1.73) | 22 | 1.31 (0.76–1.85) | |
| Mali | 2,447 (8.3) | 187 | 2.45 (2.10–2.81) | 202 | 2.53 (2.18–2.88) | |
| Nigeria | 8,711 (29.6) | 800 | 3.43 (3.19–3.67) | 824 | 3.43 (3.19–3.66) | |
| Senegal | 287 (1.0) | 10 | 2.40 (0.91–3.89) | 11 | 2.51 (1.03–4.00) | |
| Togo | 1,437 (4.9) | 68 | 3.29 (2.51–4.07) | 71 | 3.35 (2.57–4.13) | |
| Follow-up post-ART (years) | ||||||
| 1st year on ART | 7,940 (27.0) | 574 | 2.36 (2.16–2.55) | 576 | 2.36 (2.16–2.55) | |
| 2nd year on ART | 5,403 (18.4) | 563 | 2.93 (2.69–3.17) | 596 | 3.17 (2.91–3.42) | |
| 3rd year on ART | 4,560 (15.5) | 399 | 2.77 (2.50–3.04) | 439 | 3.18 (2.89–3.48) | |
| 4th year on ART | 3,358 (11.4) | 318 | 3.08 (2.74–3.41) | 371 | 3.82 (3.43–4.21) | |
| 5th year on ART and more | 8,164 (27.8) | 450 | 2.92 (2.65–3.19) | 533 | 3.67 (3.36–3.99) | |
| Age at pregnancy (years) | ||||||
| 40–49 | 9,232 (31.4) | 118 | 0.53 (0.43–0.62) | 128 | 0.57 (0.47–0.66) | |
| 35–39 | 6,729 (22.9) | 523 | 2.74 (2.50–2.97) | 567 | 2.79 (2.56–3.02) | |
| 30–34 | 7,298 (24.8) | 896 | 4.17 (3.89–4.44) | 991 | 4.29 (4.03–4.56) | |
| 25–29 | 4,364 (14.8) | 618 | 4.69 (4.32–5.06) | 671 | 4.78 (4.42–5.15) | |
| 20–24 | 1,170 (4.0) | 141 | 4.01 (3.35–4.68) | 150 | 4.11 (3.45–4.76) | |
| 16–19 | 126 (0.4) | 8 | 1.95 (0.60–3.30) | 8 | 1.92 (0.59–3.26) | |
| missing | 506 (1.7) | - | - | |||
| Baseline clinical stage | ||||||
| A, B/I, II | 18,191 (61.8) | 1,574 | 3.20 (3.04–3.35) | 1,712 | 3.30 (3.14–3.45) | |
| C/III, IV | 7,100 (24.1) | 452 | 2.33 (2.11–2.54) | 500 | 2.45 (2.23–2.66) | |
| Missing | 4,134 (14.1) | 278 | 2.30 (2.03–2.57) | 303 | 2.42 (2.15–2.69) | |
| Baseline CD4 count (cells/µl) | ||||||
| <50 | 3,399 (11.6) | 194 | 2.24 (1.93–2.56) | 215 | 2.39 (2.07–2.71) | |
| 50–99 | 2,838 (9.6) | 193 | 2.53 (2.17–2.88) | 220 | 2.74 (2.38–3.10) | |
| 100–199 | 6,290 (21.4) | 527 | 3.01 (2.75–3.27) | 574 | 3.10 (2.85–3.36) | |
| 200–299 | 4,829 (16.4) | 430 | 3.34 (3.02–3.66) | 463 | 3.41 (3.10–3.72) | |
| 300–349 | 1,446 (4.9) | 117 | 3.38 (2.77–4.00) | 120 | 3.31 (2.71–3.90) | |
| 350–499 | 1,596 (5.4) | 143 | 3.57 (2.99–4.16) | 154 | 3.63 (3.06–4.21) | |
| ≥500 | 1,320 (4.5) | 119 | 3.72 (3.05–4.38) | 126 | 3.73 (3.08–4.38) | |
| Missing | 7,707 (26.2) | 581 | 2.48 (2.28–2.68) | 643 | 2.62 (2.42–2.82) | |
| First ART regimen | ||||||
| 2NRTIs+NVP | 18,249 (62.0) | 1,665 | 3.48 (3.31–3.65) | 1,795 | 3.56 (3.40–3.73) | |
| 2NRTIs+EFV | 8,108 (27.6) | 416 | 1.75 (1.58–1.91) | 468 | 1.88 (1.71–2.05) | |
| 2NRTIs+PI | 2,271 (7.7) | 156 | 2.41 (2.03–2.79) | 179 | 2.60 (2.22–2.98) | |
| 3NRTIs/Others | 797 (2.7) | 67 | 2.57 (1.96–3.19) | 73 | 2.67 (2.06–3.28) | |
| History of ART | ||||||
| Naïve | 27,489 (93.4) | 2,142 | 2.86 (2.73–2.98) | 2,337 | 2.97 (2.85–3.09) | |
| Pre-treated | 1,936 (6.6) | 162 | 2.82 (2.39–3.25) | 178 | 2.93 (2.50–3.36) | |
| Baseline hemoglobin (g/dl) | ||||||
| ≥12 | 2,634 (9.0) | 217 | 3.11 (2.69–3.52) | 232 | 3.15 (2.75–3.56) | |
| [10–12[ | 6,917 (23.5) | 629 | 3.28 (3.02–3.54) | 698 | 3.45 (3.19–3.70) | |
| [8–10[ | 5,610 (19.1) | 455 | 2.94 (2.67–3.21) | 494 | 3.01 (2.75–3.28) | |
| <8 | 2,217 (7.5) | 147 | 2.80 (2.35–3.26) | 163 | 2.96 (2.50–3.41) | |
| Missing | 12,047 (40.9) | 856 | 2.53 (2.36–2.70) | 928 | 2.63 (2.46–2.80) | |
| Total cohort | 2,304 | 2.85 (2.74–2.97) | 2,515 | 2.96 (2.85–3.08) | ||
95% confidence interval
ART: antiretroviral therapy
EFV: efavirenz
NVP: nevirapine
NRTI: nucleoside reverse transcriptase inhibitor
PI: protease inhibitor
Median time from ART initiation to the first pregnancy was 24.6 months (IQR: 12.1–43.1). Among women reporting several pregnancies, the median time between first pregnancy and the second one was 19.7 months (IQR: 13.1–30.3) and 15.3 months (IQR: 14.1–22.1) between the second and the third ones.
As shown also in Table 2, the incidence rate of first pregnancy was 2.85 per 100 women-years (95% CI: 2.74–2.97) during the first 12 months following ART initiation and was increasing thereafter. The incidence rate of pregnancy was higher than four pregnancies per 100 women-year among women aged between 20 and 34 years, especially among those aged between 25 and 29 years peaking at 4.69 pregnancies per 100 women-years (95% CI: 4.32 – 5.06). For age groups 35 to 39 years old and 16 to 19 years old incidence rate of pregnancy was of 2.74 (95% CI: 2.50 – 2.97) and 1.95 (95% CI: 0.60 – 3.30) pregnancies per 100 women-year, respectively. The lowest incidence rate of pregnancy was estimated in the age group of 40 to 49 years old (0.53 pregnancies per 100 women-year (95%CI: 0.43 – 0.62).
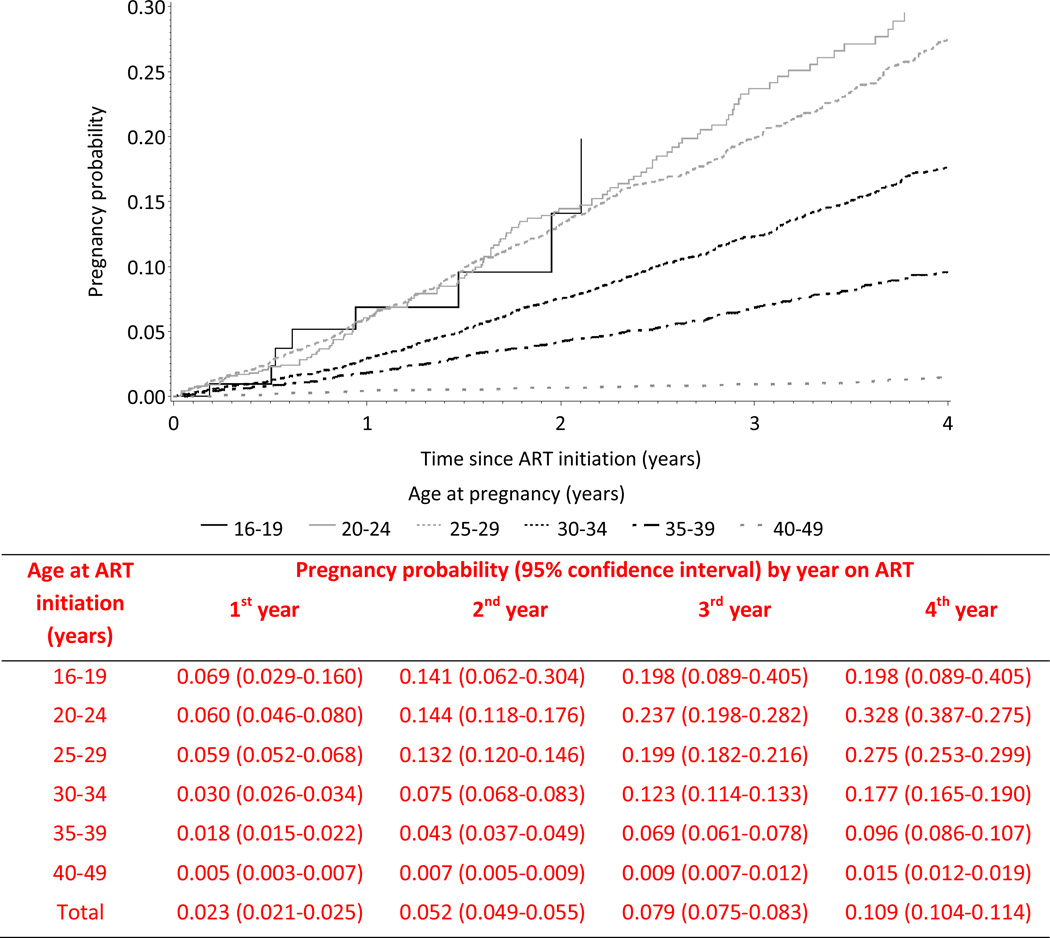
As shown in Figure 2, the overall pregnancy probability was 11% by the fourth year on ART; between 19 and 33% among women aged <30 years at ART initiation, around 18% among women aged 30 to 35 years, and much lower in older women.
Figure 2.
Kaplan-Meier probability of pregnancy occurrence after antiretroviral therapy (ART) initiation according to age at ART initiation. The IeDEA West Africa Collaboration, 1998–2011.
As presented in Table 2, incidence rate of first pregnancy after ART initiation among women starting ART with a clinical stage A,B/I,II was 3.20 per 100 women-year (95% CI: 3.04–3.35) whereas this figure was lower among women starting ART at more advanced stages of HIV disease. Similarly, the incidence rate of first pregnancy among women starting ART with CD4 cells counts of ≥500 cells/mm3 was 3.72 per 100 women-years (95% CI: 3.05–4.38) and decreased proportionally to the severity of immune deficiency at ART initiation. Although not statistically significant, pregnancy tended to be less frequent among women starting ART with lower levels of hemoglobin (Table 2).
Factors associated with pregnancy occurrence
The following factors collected at ART initiation and during follow-up were investigated in multivariable regression analysis to estimate adjusted incidence risk ratios (aIRR) of the first pregnancy: country, year of starting ART, age (time dependent variable), clinical stage, baseline CD4-cell count, ART regimen, history of ART and hemoglobin (Table 3).
Table 3.
Poisson regression univariable and multivariable model estimating associated factors of the incidence rate of first pregnancy after antiretroviral therapy (ART) initiation (29,425 women; 84,870 person-years). The IeDEA West Africa Collaboration, 1998–2011.
| Predicting factors | Number of reported pregnancies (1st event) |
Univariable models | Multivariable model | ||
|---|---|---|---|---|---|
|
Incidence rate ratio (95% CI) |
p-value |
Adjusted incidence rate ratio (95% CI) |
p-value | ||
| Country | <0.0001 | 0.0006 | |||
| Côte d’Ivoire | 777 | Ref | Ref | ||
| Benin | 101 | 0.88 (0.71–1.08) | 0.92 (0.74–1.14) | ||
| Burkina Faso | 341 | 1.32 (1.16–1.50) | 1.24 (1.09–1.42) | ||
| Guinea Bissau | 20 | 0.48 (0.31–0.75) | 0.59 (0.38–0.93) | ||
| Mali | 187 | 0.98 (0.83–1.15) | 0.90 (0.76–1.08) | ||
| Nigeria | 800 | 1.37 (1.24–1.51) | 0.99 (0.89–1.11) | ||
| Senegal | 10 | 0.96 (0.51–1.79) | 1.01 (0.54–1.9) | ||
| Togo | 68 | 1.30 (1.01–1.67) | 0.83 (0.64–1.08) | ||
| Year of starting ART | <0.0001 | <0.0001 | |||
| <2005 | 299 | Ref | Ref | ||
| 2005–2006 | 722 | 1.24 (1.09–1.42) | 0.89 (0.77–1.04) | ||
| 2007–2008 | 760 | 1.83 (1.60–2.09) | 1.34 (1.15–1.56) | ||
| 2009–2011 | 523 | 2.16 (1.87–2.49) | 1.58 (1.35–1.86) | ||
| Age (years, time dependant) | <0.0001 | <0.0001 | |||
| 40–49 | 118 | Ref | Ref | ||
| 35–39 | 523 | 5.26 (4.31–6.42) | 5.11 (4.19–6.24) | ||
| 30–34 | 896 | 8.00 (6.60–9.69) | 7.64 (6.30–9.26) | ||
| 25–29 | 618 | 9.00 (7.39–10.96) | 8.26 (6.77–10.07) | ||
| 20–24 | 141 | 7.71 (6.04–9.85) | 7.12 (5.57–9.11) | ||
| 16–19 | 8 | 3.74 (1.83–7.66) | 3.52 (1.72–7.20) | ||
| Clinical stage at ART initiation | <0.0001 | <0.0001 | |||
| A, B/I, II | 1,574 | Ref | Ref | ||
| C/III, IV | 452 | 0.73 (0.66–0.81) | 0.83 (0.74–0.93) | ||
| Missing | 278 | 0.72 (0.64–0.82) | 0.78 (0.68–0.90) | ||
| CD4 count at ART initiation (cells/µl) | <0.0001 | 0.0032 | |||
| <50 | 194 | Ref | Ref | ||
| 50–99 | 193 | 1.13 (0.92–1.37) | 1.14 (0.93–1.39) | ||
| 100–199 | 527 | 1.34 (1.14–1.58) | 1.25 (1.06–1.48) | ||
| 200–299 | 430 | 1.48 (1.25–1.76) | 1.38 (1.16–1.64) | ||
| 300–349 | 117 | 1.50 (1.19–1.88) | 1.35 (1.07–1.70) | ||
| 350–499 | 143 | 1.58 (1.27–1.96) | 1.41 (1.13–1.76) | ||
| ≥500 | 119 | 1.64 (1.31–2.06) | 1.43 (1.13–1.81) | ||
| Missing | 581 | 1.10 (0.94–1.30) | 1.38 (1.15–1.66) | ||
| Regimen at ART initiation | <0.0001 | <0.0001 | |||
| 2NRTIs+NVP | 1,665 | Ref | Ref | ||
| 2NRTIs+EFV | 416 | 0.50 (0.45–0.56) | 0.63 (0.56–0.71) | ||
| 2NRTIs+PI | 156 | 0.69 (0.59–0.81) | 0.86 (0.72–1.02) | ||
| 3NRTIs/others | 67 | 0.74 (0.58–0.95) | 0.96 (0.75–1.23) | ||
| Hemoglobin at ART initiation (g/dl) | <0.0001 | 0.0012 | |||
| ≥12 | 217 | Ref | Ref | ||
| [10–12[ | 629 | 0.94 (0.80–1.11) | 1.14 (0.97–1.35) | ||
| [8–10[ | 455 | 0.90 (0.73–1.11) | 1.11 (0.90–1.38) | ||
| <8 | 147 | 1.05 (0.90–1.23) | 1.15 (0.98–1.34) | ||
| Missing | 856 | 0.81 (0.70–0.94) | 0.90 (0.76–1.06) | ||
| ART history | 0.8805 | ||||
| ART naïve | 2,142 | Ref | - | ||
| Pre-treated | 162 | 0.99 (0.84–1.16) | - | ||
CI : confidence interval
EFV : efavirenz
NVP : nevirapine
NRTI : nucléoside reverse transcriptase inhibitor
PI : protease inhibitor
When compared to women aged 40–49 years, women aged 16–19, 20–24, 25–29, 30–34 and 35–39 years had an increased risk of becoming pregnant after ART initiation (aIRR of 3.52, 7.12, 8.26, 7.64 and 5.11, respectively, p <0.001).
Starting ART at an advanced clinical stage of HIV disease (CDC stages C or WHO stage III and IV) reduced significantly the likelihood of pregnancy after ART initiation (aIRR: 0.83; 95% CI: 0.74–0.93). CD4-cell count at ART initiation discriminated well the risk of first pregnancy after ART initiation: the higher the CD4 count at ART initiation, the more frequent the subsequent occurrence of pregnancy. Indeed, compared to women starting ART with a CD4-cell count under 50 cells/mm3 the aIRR of pregnancy post-ART initiation was 1.14 (95% CI: 0.93–1.39), 1.25 (95% CI: 1.06–1.48), 1.38 (95% CI: 1.16–1.64), 1.35 (95% CI: 1.07–1.70), 1.41 (95% CI: 1.13–1.76) and 1.43 (95% CI: 1.13–1.81) for women starting with CD4 cell count between 50–99 cells/mm3, 100–199 cells/mm3, 200–299 cells/mm3, 300–349 cells/mm3, 350–499 cells/mm3 and ≥500 cells/mm3, respectively.
Finally, when compared to those initiating ART with a Nevirapine-based regimen, HIV-infected women starting ART with an Efavirenz-based regimen had a significantly decreased aIRR (0.63, 95% CI: 0.56–0.71). The previous history of ARV use did not influence the pregnancy occurrence after ART initiation.
Discussion
This study estimated the occurrence of pregnancy among HIV-infected women after ART initiation at 15 clinical sites in eight West African countries over a 10 year period. In order to explain demographic trends of the incidence of pregnancy in this population, we looked as well for epidemiological, clinical, biological and therapeutic characteristics. According to our findings, the average crude incidence rate of pregnancy after ART initiation was 2.9 pregnancies per 100 women-years. To the best of our knowledge, it is the first time that such a figure is reported in this part of Africa known for its high fertility patterns and infrequent use of contraceptive methods. Although elevated, the estimated pregnancy incidence we found in this population living with HIV and in care in West Africa was lower than among their uninfected counterparts in the region. Indeed, according to the 2012 African health observatory report, fertility rates across the countries we surveyed ranged between 4 and 6 livebirths per 1000 women-year(25). Although this variation might be owed to methodological differences, it may also suggest that fertility patterns are somehow conditioned by HIV infection.
Similarly, it appeared that the incidence rate of pregnancy within our study sample was lower than previous estimations in other sub-Saharan African settings. In Southern Africa, Bussmann et al. reported a post-ART pregnancy rate of 7.9 per 100 women-years in Botswana (26) and Westreich et al. reported an incidence rate of pregnancies of 5.2 per 100 women-years after ART initiation in a South African clinical cohort (15). Furthermore, in rural Uganda, the incidence rate of pregnancy was as high as 9.5 per 100 women-years 24 months after ART initiation (13). Finally, a larger study conducted by Myer et al. reported an overall crude incidence rate of pregnancy after ART initiation of 9.0 per 100 women-years in seven African countries including Côte d’Ivoire. Although not fully detailed, Myers et al estimation for Côte d’Ivoire appeared higher than the one we found in our study. We suspect that this difference might be owed to the fact that their study was conducted within a network of women-centered clinics focusing on PMTCT of HIV infection, thus pregnancy was the main outcome measure with probably more accurate and rigorous detection methods (14).
We also suspect that differences in occurrence of pregnancy after ART Initiation between West Africa and other African settings might be due to socio-demographic specificities and HIV disease and care patterns. Methodological variations across studies cannot however be fully excluded. Although systematically confirmed clinically, estimations on incidence rate of pregnancy presented in our study were initially based on women self-report of the last menstruation period. Bearing in mind that miscarriages and abortions are very frequent during the early months of pregnancy (27) and HIV infection has been pointed out as an important cause (28), we suspect that a substantial number of pregnancies may have gone undetected. The former and the significant proportion of dropouts contributed clearly to an underestimation of the pregnancy incidence reported. Similarly, in this context of large-scale public health program, the data quality did not allow to document how patients break in care in a standardized way across sites. Finally, the lack of information on some potentially important confounding variables (such as marital status, parity or family planning methods availability or use) limits somewhat the interpretation of our findings.
The incidence rate of pregnancy after ART initiation increased slightly but progressively throughout years in care, suggesting a positive effect of ART on fertility among women of reproductive age, and this association was particularly strong among younger women. This phenomenon is consistent with studies conducted in other sub-Saharan African settings, showing an increased cumulative incidence rate of pregnancy post-ART initiation proportional to time on clinical follow-up (14, 15, 26). This finding suggests that the global restoration of health status favored by ART and measured by CD4 cell recovery also boosted fertility functions among women of reproductive age. It is also possible that immune restoration owed to ART favored the onset of maternal-fetus tolerance mechanism increasing the probability to take a pregnancy to term successfully. However, such a biological association between post-ART health improvement and restoration of fertility functions requires further research to better understand the underlying biological mechanisms and their association with temporal changes in couple and family life experiences.
Additionally, our findings show that both age and clinical/immunological parameters at ART initiation were important predictors of reproductive patterns among HIV-infected women in care. Consistently with other studies, the incidence rate ratio of pregnancy after ART initiation was significantly higher among younger and healthier HIV-infected women than among their older counterparts with a poorer health status (13–15). However the increase of the incidence rate of pregnancy after ART initiation reached its peak at 25–29 years old to decrease progressively afterwards, suggesting that although ART might have a positive effect on fertility, it remains restricted to biological conditions, respecting human reproductive cycle.
Healthier women at ART initiation were those having the highest probability of becoming pregnant. This association is explained by the fact that their improved health status increases their reproductive ability but also by the fact that an early exposure to ART might limit the HIV-associated risk of miscarriage. This increased incidence risk of pregnancy associated to higher CD4 levels at ART initiation should be better taken into account in strategies such as treatment as prevention promoting universal and early treatment initiation regardless of CD4 levels.
Unintended pregnancy rates are high in sub-Saharan Africa among women of reproductive age and raise major public health concerns among younger women in these settings (29). We hypothesize that besides the potential benefits of ART on restoring fertility functions, an important number of these pregnancies are owed to the limited capacity of HIV-infected women to manage their procreation desires. Indeed, the unmet needs for family planning among west-African women irrespective of their HIV status is as high as 30% (9, 30). It has been pointed out that integrating family planning services into HIV care is an important shortcoming to reduce unintended pregnancy rates among HIV-infected women and eliminate the vertical risk of HIV transmission (1, 31, 32).
To conclude, this is the one of the first studies estimating incidence rate of pregnancy among a large regional cohort of about 30,000 HIV-infected women initiating ART for their own health and accounting for about 75,000 women-years of follow-up across eight West African countries. Although lower than in other African settings and probably underestimated, the incidence rate of pregnancy increases proportionally after ART initiation in West Africa. Moreover, pregnancy after ART initiation remains an event, particularly for younger women, with potentially important individual and public health consequences. As universal access to ART has enabled HIV-infected women of reproductive age to live longer and with higher quality of life standards, childbearing is a growing desire among this population. Understanding the dynamics of fertility among women on ART is a key step to correctly fit the strategies of integrating family planning into HIV care and to help HIV-infected women to correctly fulfill their procreation trajectory through safe and adapted motherhoods programs. This is one of the many challenges that HIV care and treatment programs must address upfront now, especially in West Africa.
Acknowledgment
We wish to warmly thank the staff of the clinical centers participating in the IeDEA West Africa collaboration for their invaluable assistance in conducting the study. We are indebted to the patients who participated in the IeDEA West Africa Collaboration.
Funding
This study was funded through the National Cancer Institute (NCI), the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) and the National Institute of Allergy and Infectious Diseases (NIAID) of the U.S. National Institutes of Health (NIH), as part of the International Epidemiologic Databases to Evaluate AIDS (IeDEA) under Award Number U01AI069919. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
J.B-S. was a PhD fellow of the French Ministry of Education, Research and Technology.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Appendix
The IeDEA West Africa Collaboration Study Group (as of November, 2013):
Participating sites (*members of the Steering Committee):
Executive Committee: François Dabis (Principal Investigator, Bordeaux, France), Emmanuel Bissagnene (Co-Principal Investigator, Abidjan, Côte d’Ivoire), Elise Arrivé (Bordeaux, France), Patrick Coffie (Abidjan, Côte d’Ivoire), Didier Ekouevi (Abidjan, Côte d’Ivoire), Antoine Jaquet (Bordeaux, France), Valériane Leroy (Bordeaux, France), Charlotte Lewden (Bordeaux, France), Annie J Sasco (Bordeaux, France).
Benin, Cotonou:
Adults: Djimon Marcel Zannou*, Carin Ahouada, Jocelyn Akakpo, Christelle Ahomadegbé, Jules Bashi, Alice Gougounon-Houéto, Angèle Azon-Kouanou, Fabien Houngbé, Jean Sehonou (CNHU Hubert Maga).
Pediatrics: Sikiratou Koumakpaï*§, Florence Alihonou, Marcelline d’Almeida, Irvine Hodonou, Ghislaine Hounhoui, Gracien Sagbo, Leïla Tossa-Bagnan, Herman Adjide (CNHU Hubert Maga).
Burkina Faso:
Adults: Joseph Drabo*, René Bognounou, Arnaud Dienderé, Eliezer Traore, Lassane Zoungrana, Béatrice Zerbo (CHU Yalgado, Ouagadougou), Adrien Bruno Sawadogo*§, Jacques Zoungrana, Arsène Héma, Ibrahim Soré, Guillaume Bado, Achille Tapsoba (CHU Souro Sanou, Bobo Dioulasso)
Pediatrics: Diarra Yé*, Fla Kouéta, Sylvie Ouedraogo, Rasmata Ouédraogo, William Hiembo, Mady Gansonré (CH Charles de Gaulle, Ouagadougou).
Côte d’Ivoire, Abidjan:
Adults: Eugène Messou*, Joachim Charles Gnokoro, Mamadou Koné, Guillaume Martial Kouakou, (ACONDA-CePReF); Clarisse Amani Bosse*, Kouakou Brou, Achi Isidore Assi (ACONDA-MTCT-Plus); Henri Chenal*, Denise Hawerlander, Franck Soppi (CIRBA); Albert Minga*, Yao Abo, Jean-Michel Yoboue (CMSDS/CNTS); Serge Paul Eholié*§, Mensah Deborah Noelly Amego, Viviane Andavi, Zelica Diallo, Frédéric Ello, Aristophane Koffi Tanon (SMIT, CHU de Treichville), Serge Olivier Koule*, Koffi Charles Anzan, Calixte Guehi (USAC, CHU de Treichville);.
Pediatrics: Edmond Addi Aka*, Koffi Ladji Issouf, Jean-Claude Kouakou, Marie-Sylvie N’Gbeche, (ACONDA-CePReF); Touré Pety*, Divine Avit-Edi (ACONDA-MTCT-Plus); Kouadio Kouakou*, Magloire Moh, Valérie Andoblé Yao (CIRBA); Madeleine Amorissani Folquet*, Marie-Evelyne Dainguy, Cyrille Kouakou, Véronique Tanoh Méa-Assande, Gladys Oka-Berete, Nathalie Zobo, Patrick Acquah, Marie-Berthe Kokora (CHU Cocody); Tanoh François Eboua*, Marguerite Timité-Konan, Lucrèce Diecket Ahoussou, Julie Kebé Assouan, Mabéa Flora Sami, Clémence Kouadio (CHU Yopougon).
Ghana, Accra:
Pediatrics: Lorna Renner*§, Bamenla Goka, Jennifer Welbeck, Adziri Sackey, Seth Ntiri Owiafe (Korle Bu TH).
Guinea-Bissau:
Adults: Christian Wejse*§, Zacarias José Da Silva*, Joao Paulo (Bandim Health Project), The Bissau HIV cohort study group: Amabelia Rodrigues (Bandim Health Project), David da Silva (National HIV program Bissau), Candida Medina (Hospital National Simao Mendes, Bissau), Ines Oliviera-Souto (Bandim Health Project), Lars Østergaard (Dept of Infectious Diseases, Aarhus University Hospital), Alex Laursen (Dept of Infectious Diseases, Aarhus University Hospital), Morten Sodemann (Dept of Infectious Diseases, Odense University Hospital), Peter Aaby (Bandim Health Project), Anders Fomsgaard (Dept. of Virology, Statens Serum Institut, Copenhagen), Christian Erikstrup (Dept. of Clinical Immunology), Jesper Eugen-Olsen (Dept. of Infectious Diseases, Hvidovre Hospital, Copenhagen).
Mali, Bamako:
Adults: Moussa Y Maïga*§, Fatoumata Fofana Diakité, Abdoulaye Kalle, Drissa Katile (CH Gabriel Toure), Hamar Alassane Traore*, Daouda Minta*, Tidiani Cissé, Mamadou Dembelé, Mohammed Doumbia, Mahamadou Fomba, Assétou Soukho Kaya, Abdoulaye M Traoré, Hamady Traoré, Amadou Abathina Toure (CH Point G).
Pediatrics: Fatoumata Dicko*, Mariam Sylla, Alima Berthé, Hadizatou Coulibaly Traoré, Anta Koïta, Niaboula Koné, Clémentine N'Diaye, Safiatou Touré Coulibaly, Mamadou Traoré, Naïchata Traoré (CH Gabriel Toure).
Nigeria:
Adults: Man Charurat* (UMB/IHV), Samuel Ajayi*, Georgina Alim, Stephen Dapiap, Otu (UATH, Abuja), Festus Igbinoba (National Hospital Abuja), Okwara Benson*, Clément Adebamowo*, Jesse James, Obaseki, Philip Osakede (UBTH, Benin City), John Olasode (OATH, Ile-Ife).
Senegal, Dakar:
Adults: Moussa Seydi*, Papa Salif Sow, Bernard Diop, Noël Magloire Manga, Judicael Malick Tine§, Coumba Cissé Bassabi (SMIT, CHU Fann),
Pediatrics: Haby Signate Sy*, Abou Ba, Aida Diagne, Hélène Dior, Malick Faye, Ramatoulaye Diagne Gueye, Aminata Diack Mbaye (CH Albert Royer).
Togo, Lomé:
Adults: Akessiwe Patassi*, Awèrou Kotosso, Benjamin Goilibe Kariyare, Gafarou Gbadamassi, Agbo Komi, Kankoé Edem Mensah-Zukong, Pinuwe Pakpame (CHU Tokoin/Sylvanus Olympio).
Pediatrics: Koko Lawson-Evi*§, Yawo Atakouma, Elom Takassi, Améyo Djeha, Ayoko Ephoévi-gah, Sherifa El-Hadj Djibril (CHU Tokoin/Sylvanus Olympio).
Operational and Statistical Team: Jean-Claude Azani (Abidjan, Côte d’Ivoire), Eric Balestre (Bordeaux, France), Serge Bessekon (Abidjan, Côte d’Ivoire), Sophie Karcher (Bordeaux, France), Jules Mahan Gonsan (Abidjan, Côte d’Ivoire), Jérôme Le Carrou (Bordeaux, France), Séverin Lenaud (Abidjan, Côte d’Ivoire), Célestin Nchot (Abidjan, Côte d’Ivoire), Karen Malateste (Bordeaux, France), Amon Roseamonde Yao (Abidjan, Côte d’Ivoire).
Administrative Team: Madikona Dosso (Abidjan, Côte d’Ivoire), Alexandra Doring§ (Bordeaux, France), Adrienne Kouakou (Abidjan, Côte d’Ivoire), Elodie Rabourdin (Bordeaux, France), Jean Rivenc (Pessac, France).
Consultants/Working Groups: Xavier Anglaret (Bordeaux, France), Boubacar Ba (Bamako, Mali), Renaud Becquet (Bordeaux, France), Juan Burgos Soto (Bordeaux, France), Jean Bosco Essanin (Abidjan), Andrea Ciaranello (Boston, USA), Sébastien Datté (Abidjan, Côte d’Ivoire), Sophie Desmonde (Bordeaux, France), Jean-Serge Elvis Diby (Abidjan, Côte d’Ivoire), Geoffrey S.Gottlieb* (Seattle, USA), Apollinaire Gninlgninrin Horo (Abidjan, Côte d’Ivoire), Julie Jesson (Bordeaux, France), Serge N'zoré Kangah (Abidjan, Côte d’Ivoire), David Meless (Abidjan, Côte d’Ivoire), Aida Mounkaila-Harouna (Bordeaux, France), Camille Ndondoki (Bordeaux, France), Caroline Shiboski (San Francisco USA), Boris Tchounga (Abidjan, Côte d’Ivoire), Rodolphe Thiébaut (Bordeaux, France), Gilles Wandeler (Dakar, Senegal).
Footnotes
Authors contributions
J.B-S. and R.B led the process of methodological design, statistical analysis and data interpretation, wrote and revised the manuscript; E.B. was in charge of data management and statistical analysis; A.M. coordinated field activities and contributed to methodological design, data interpretation and paper writing; S.A., A.S. and M.Z. managed field work and participated in data interpretation; V.L. and D.K.E. contributed to methodological design, and participated in data interpretation and paper writing; F.D. was the primary investigator of the IeDEA West Africa Collaboration, contributed to methodological design, and participated in data interpretation, paper writing and revision.
Previous presentation
This study was presented in part in February 2014 at the 2014 Conference on Retrovirus and Opportunistic Infections (CROI), Boston, USA (poster #868).
Conflict of interest
None of the authors has any conflict of interest to declare (as per the Unified Competing Interest form).
References
- 1.UNAIDS. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS (UNAIDS); 2012. UNAIDS Report on the global AIDS epidemic. [Google Scholar]
- 2.UNAIDS. 2013 progress report on the global plan. Towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive. 2013 doi: 10.7448/IAS.15.4.17390. [DOI] [PMC free article] [PubMed]
- 3.Mmbaga EJ, Leyna GH, Ezekiel MJ, Kakoko DC. Fertility desire and intention of people living with HIV/AIDS in Tanzania: a call for restructuring care and treatment services. BMC Public Health. 2013;13:86. doi: 10.1186/1471-2458-13-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badell ML, Lathrop E, Haddad LB, Goedken P, Nguyen ML, Cwiak CA. Reproductive healthcare needs and desires in a cohort of HIV-positive women. Infect Dis Obstet Gynecol. 2012;2012:107–878. doi: 10.1155/2012/107878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mmeje O, Cohen CR, Cohan D. Evaluating safer conception options for HIV-serodiscordant couples (HIV-infected female/HIV-uninfected male): a closer look at vaginal insemination. Infect Dis Obstet Gynecol. 2012;2012:587–651. doi: 10.1155/2012/587651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marie Stopes International. Global Impact Report 2011 Delivering choice and rights for women: past, present and future. London, England: 2011. [Google Scholar]
- 7.WHO, UNICEF. The 2012 report. Geneva: 2012. Countdown to 2015. Maternal, newborn & child survival. Building a future for women and children. [Google Scholar]
- 8.Cleland JG, Ndugwa RP, Zulu EM. Family planning in sub-Saharan Africa: progress or stagnation? Bull World Health Organ. 2011 Feb 1;89(2):137–143. doi: 10.2471/BLT.10.077925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marie Stopes International. Family planning: Francophone West-Africa on the move. A call to action. Ouagadougou, Burkina Faso: Marie Stopes International; 2011. [Google Scholar]
- 10.USAID. Report of September 2012 Bellagio consultation. New York: A fine balance: Contraceptive choice in the 21st century - an action agenda. [Google Scholar]
- 11.Nations U. The Millenium Development Goals Report 2013. New York, USA: 2013. [Google Scholar]
- 12.Berhan Y, Berhan A. Meta-analyses of fertility desires of people living with HIV. BMC Public Health. 2013 Apr 30;13(1):409. doi: 10.1186/1471-2458-13-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Homsy J, Bunnell R, Moore D, King R, Malamba S, Nakityo R, et al. Reproductive intentions and outcomes among women on antiretroviral therapy in rural Uganda: a prospective cohort study. PLoS One. 2009;4(1):e4149. doi: 10.1371/journal.pone.0004149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Myer L, Carter RJ, Katyal M, Toro P, El-Sadr WM, Abrams EJ. Impact of antiretroviral therapy on incidence of pregnancy among HIV-infected women in Sub-Saharan Africa: a cohort study. PLoS Med. 2010 Feb;7(2):e1000229. doi: 10.1371/journal.pmed.1000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Westreich D, Maskew M, Rubel D, MacDonald P, Jaffray I, Majuba P. Incidence of pregnancy after initiation of antiretroviral therapy in South Africa: a retrospective clinical cohort analysis. Infect Dis Obstet Gynecol. 2012;2012:917059. doi: 10.1155/2012/917059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Westreich D, Cole SR, Nagar S, Maskew M, van der Horst C, Sanne I. Pregnancy and virologic response to antiretroviral therapy in South Africa. PLoS One. 2011;6(8):e22778. doi: 10.1371/journal.pone.0022778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaba B, Calvert C, Marston M, Isingo R, Nakiyingi-Miiro J, Lutalo T, et al. Effect of HIV infection on pregnancy-related mortality in sub-Saharan Africa: secondary analyses of pooled community-based data from the network for Analysing Longitudinal Population-based HIV/AIDS data on Africa (ALPHA) Lancet. 2013 May 18;381(9879):1763–1771. doi: 10.1016/S0140-6736(13)60803-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet. 2006 Apr 1;367(9516):1066–1074. doi: 10.1016/S0140-6736(06)68397-9. [DOI] [PubMed] [Google Scholar]
- 19.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012 Dec 15;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthews LT, Kaida A, Kanters S, Byakwagamd H, Mocello AR, Muzoora C, et al. HIV-infected women on antiretroviral treatment have increased mortality during pregnant and postpartum periods. AIDS. 2013 Oct;27(Suppl 1):S105–S112. doi: 10.1097/QAD.0000000000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suy A, Martinez E, Coll O, Lonca M, Palacio M, de Lazzari E, et al. Increased risk of preeclampsia and fetal death in HIV-infected pregnant women receiving highly active antiretroviral therapy. AIDS. 2006 Jan 2;20(1):59–66. doi: 10.1097/01.aids.0000198090.70325.bd. [DOI] [PubMed] [Google Scholar]
- 22.Powis KM, McElrath TF, Hughes MD, Ogwu A, Souda S, Datwyler SA, et al. High viral load and elevated angiogenic markers associated with increased risk of preeclampsia among women initiating highly active antiretroviral therapy in pregnancy in the Mma Bana study, Botswana. J Acquir Immune Defic Syndr. 2013 Apr 15;62(5):517–524. doi: 10.1097/QAI.0b013e318286d77e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaffer N, Abrams EJ, Becquet R. Option B+ for prevention of mother-to-child transmission of HIV in resource-constrained settings: great promise but some early caution. Aids. 2014 Feb 20;28(4):599–601. doi: 10.1097/QAD.0000000000000144. [DOI] [PubMed] [Google Scholar]
- 24.Egger M, Ekouevi DK, Williams C, Lyamuya RE, Mukumbi H, Braitstein P, et al. Cohort Profile: the international epidemiological databases to evaluate AIDS (IeDEA) in sub-Saharan Africa. Int J Epidemiol. 2012 Oct;41(5):1256–1264. doi: 10.1093/ije/dyr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO. Atlas of health statistics of the african region 2012. Health situation analysis of the african region. 2012 [Google Scholar]
- 26.Bussmann H, Wester CW, Wester CN, Lekoko B, Okezie O, Thomas AM, et al. Pregnancy rates and birth outcomes among women on efavirenz-containing highly active antiretroviral therapy in Botswana. J Acquir Immune Defic Syndr. 2007 Jul 1;45(3):269–273. doi: 10.1097/QAI.0b013e318050d683. [DOI] [PubMed] [Google Scholar]
- 27.Wilcox AJ, Weinberg CR, O'Connor JF, Baird DD, Schlatterer JP, Canfield RE, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988 Jul 28;319(4):189–194. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- 28.Kolte L, Gaardbo JC, Karlsson I, Sorensen AL, Ryder LP, Skogstrand K, et al. Dysregulation of CD4+CD25+CD127lowFOXP3+ regulatory T cells in HIV-infected pregnant women. Blood. 2011 Feb 10;117(6):1861–1868. doi: 10.1182/blood-2010-07-298992. [DOI] [PubMed] [Google Scholar]
- 29.Hubacher D, Mavranezouli I, McGinn E. Unintended pregnancy in sub-Saharan Africa: magnitude of the problem and potential role of contraceptive implants to alleviate it. Contraception. 2008 Jul;78(1):73–78. doi: 10.1016/j.contraception.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Alkema L, Kantorova V, Menozzi C, Biddlecom A. National, regional, and global rates and trends in contraceptive prevalence and unmet need for family planning between 1990 and 2015: a systematic and comprehensive analysis. Lancet. 2013 May 11;381(9878):1642–1652. doi: 10.1016/S0140-6736(12)62204-1. [DOI] [PubMed] [Google Scholar]
- 31.UNAIDS. Countdown to zero. Global Plan Towards the Elimination of New HIV Infections Among Children By 2015 And Keeping Their Mothers Alive.: Joint United Nations Programme on HIV/AIDS (UNAIDS) 2011 [Google Scholar]
- 32.Baumgartner JN, Green M, Weaver MA, Mpangile G, Kohi TW, Mujaya SN, et al. Integrating family planning services into HIV care and treatment clinics in Tanzania: evaluation of a facilitated referral model. Health Policy Plan. 2013 Jul 26; doi: 10.1093/heapol/czt043. [DOI] [PMC free article] [PubMed] [Google Scholar]